|
09-07-2025
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting
bug fixes and updates
to web eAF made in the version 1.2.0.8 released to production on 07 July 2025 are now available on PLM Portal and on the PLM Portal eAF web page
02-07-2025
Planned maintenance of eSubmission systems on 9 July 2025, 18:00 CET
Due to planned maintenance, the Common Repository Web User Interface
(relevant for NCAs only) will not be available on Wednesday 9 July 2025, between 18:00 and 20:00.
For any further information, please contact EMA Service Desk
01-07-2025
PLM Portal FHIR XML version upgrade
The PLM portal FHIR XML version was updated to 2.2.3. The new version introduces the following changes:
• There’s a new extension in (sub)Task.code.coding[system=https://spor.ema.europa.eu/v1/lists/100000152091].extension[system=http://ema.europa.eu/fhir/termName].valueString containing the full name of the scope. For example: “B.II.e.7.a Deletion of a supplier”, instead of “B.II.e.7.a”
• The DataCarrierIdentifier identifier was moved from PackagedProductDefinition.package.identifier to PackagedProductDefinition.identifier
The Release notes can be found here.
The next upgrade of the PLM Portal FHIR XML (2.2.4) is currently planned for the end of July 2025, and the date will be
confirmed in due time. The version 2.2.4 will introduce a change on the HTTP protocol of the links referenced in the FHIR
message. The recommended FHIR protocol is HTTP, therefore all the links currently using HTTPS will be changed to HTTP.
30-06-2025
eCTD v4.0 Technical Pilot - Step 2 started
The electronic Common Technical Document (eCTD) v4.0 team is pleased to launch Step
2 of the eCTD v4.0 Technical Pilot, for Centrally Authorised Products (CAP).
Please note that the participation is very limited and the participants will be selected
based on the readiness and compliance with the scenarios and focus described below.
We would like to invite interested MAHs to provide details of their proposed products,
scenarios and very importantly the name of the eCTD v4.0 tool, by 15 July by email to eCTD4consultation@ema.europa.eu.
Upon confirmation from EMA will send details on how the test packages can be submitted
Important notes
- all the test submissions from Step 2 will be processed in an EMA
Test
environment and the submission channel will not be EMA Gateway
- a first draft of the validation criteria will be published in the
upcoming weeks, and the initial test submissions of Step 2 will not go through a
“standard” validation
The main scenarios below will be followed, but please send your proposals for
different/new scenarios when expressing your interest to participate:
- Scenario 1. Initial MAA (sequence 1); EMA will
prioritise for processing
those MAHs who can resubmit sequence 1 for an existing/already authorised CAP
(previously created in eCTD 3.2.2, sequence 0000, even if currently is not a
valid
product anymore), and now re-created/converted (fully or partially) in eCTD
v4.0.
Focus on:
- controlled vocabularies
- different file formats
- multiple pack sizes, manufacturers
- Scenario 2. Initial MAA (sequence 1) for a
Duplicate product sent in scenario 1 (the Duplicate product can be a mock
product; this will facilitate early testing of Grouped submissions
functionality, which is planned for Step 3)
Focus on:
- Document reuse
- different file formats
- Scenario 3. Validation responses (starting with
sequence 2), responses
Focus on:
- Document lifecycle management (replace context of use (one to
one, one to many and many to one), delete)
- Updating keywords, priority numbers, and document titles
- Regulatory activity (Submission and related sequences for 1
regulatory activity)
- Scenario 4. Post authorisation activities
(different procedure types and multiple sequences related to the products sent
in sequence 1)
Focus on:
- Document lifecycle management (replace context of use (one to
one, one to many and many to one), delete)
- Updating keywords, priority numbers, and document titles
- Parallel regulatory activities
In Step 2 (the planned initial duration of this step is 2 months: 15 July – 15
September, subject to extension if necessary) of the eCTD v4.0 of the technical pilot
there will be no focus on Grouped submissions functionality, forward compatibility.
These will be tested in Step 3, planned for late Q3 2025.
Please note: all the communication will take place over email and
depending on the
number of test packages received and EMA eCTD v4.0 team availability, there might be a
delay in the response time.
And
Training session on Human variations web-based electronic Application
Form (eAF) for non-CAPs - 1 July 2025 10:00 - 11:30 Amsterdam time (CEST)
The registration for the training session on Human variations web-based
electronic Application Form (eAF) for non-CAPs on 1 July 2025 10:00 - 11:30
Amsterdam time (CEST) is still open. The details can be found on the event page.
Starting from end of May 2025 EMA recommends the use of the PLM Portal web-based eAF for
all non-CAPs human variations, where possible. This training aims to support users with
the adoption of the web-based eAF and to showcase new functionalities, user experience
and user interface improvement.
Video recording is made available after the event.
25-06-2025
Updated PLM Portal eAF Release notes now available
An updated version of the Portal eAF Release notes reflecting bug fixes and updates
to web eAF made in the version 1.2.0.7 released to production on 23 June 2025 are now available on
PLM Portal and on the PLM Portal eAF web page
And
Updated PLM Portal eAF guide to navigation now available
An updated draft version of the PLM Portal eAF guide to navigation is now available.
The guide to navigation contains part of the new UI/UX design, and more updates will be added in future versions of the guide.
12-06-2025
Updated PLM Portal eAF Release notes now available
An updated version of the Portal eAF Release notes reflecting bug fixes and updates
to web eAF made in the version 1.2.0.6 released to production on 10 June 2025 are now available on
PLM Portal and on the PLM Portal eAF web page
And
Planned maintenance of PSUR systems and Important notification
Due to planned maintenance, the PSUR Repository (Industry access) will not be available
today between 18:00 and 19:30.
For any further information, please contact EMA Service Desk
Reminder: Due to planned maintenance, the PSUR Repository (Industry
access) will not be functional
during 20 - 22 June 2025. During this downtime, the PSUR packages that
are submitted through the EMA gateway
will not be processed. During the week 23 - 27 June
2025, the PSUR Repository and the PSUR submissions will be
closely monitored, and even though all the potential scenarios were carefully
considered, unforeseen disruptions could occur during this
week.
To avoid any delays in sending the PSUR packages, it is highly recommended to submit the
packages a few days before the 20th of June 2025.
For further details, please follow the EMA Service Desk and
eSubmissions.
03-06-2025
Planned maintenance of PSUR systems - Important notification
Due to planned maintenance, the PSUR Repository (Industry access) will not be functional
during 20 - 22 June 2025. During this downtime, the PSUR packages that are submitted
through the EMA gateway will not be processed.
During the week 23 - 27 June 2025, the PSUR Repository and the PSUR submissions will be
closely monitored, and even though all the potential scenarios were carefully considered,
unforeseen disruptions could occur during this week.
To avoid any delays in sending the PSUR packages, it is highly recommended to submit the
packages a few days before the 20th of June 2025.
For further details, please follow the EMA Service Desk
and eSubmissions.
02-06-2025
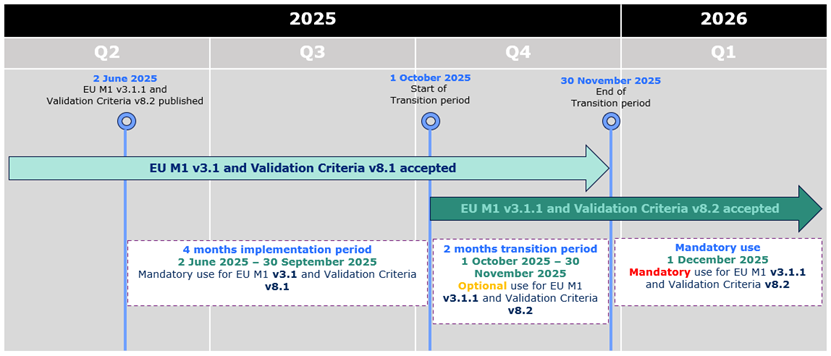
eCTD 3.2.2 - new package available
A new version of the
EU eCTD M1 Specification, version 3.1.1
,
is now published on the
eSubmission website
.
The version 3.1.1 sees the introduction of a small number of changes in the
specification, related to the tracking table for EDQM and new examples of product
numbers and procedure numbers for work-sharing and super-grouping.
The changes are reflected in the
Release Notes
.
A new version of the
validation criteria v8.2
has been published on the
eSubmission website. The version is related to the
EU Module 1 Specification version 3.1.1 and should be used in case of submitting a new
sequence according to EU M1 specification v3.1.1. The new validation criteria will
be used for the technical validation for all v3.1.1 electronic submissions received
as of 1 December 2025 to the NCAs and EMA.
The changes are reflected in the
Release Notes.
A new version of the
Harmonised guidance
eCTD 6.0.1 was published together with the new version of the specification and the validation criteria
Note: The Util files remain the same
During the initial period of 4 months from 2 June 2025 to 30 September 2025,
applicants can only submit eCTD format submissions compliant with EU M1 v3.1 and validation criteria version 8.1.
From 1 October 2025 eCTDs compliant with EU M1 v3.1 or v3.1.1 and validation criteria v8.1 or v8.2 are accepted.
From 1 December 2025 only eCTDs compliant with EU M1 v3.1.1 and validation criteria v8.2 are accepted.
27-05-2025
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting
bug fixes and updates
to web eAF made in the version 1.2.0.5 released to production on 26 May 2025 are now
available on PLM Portal and on the PLM Portal eAF web page
22-05-2025
Planned maintenance of eSubmission systems on 23 May 2025, 18:00 CET
Due to planned maintenance, the following eSubmission systems will not be available on
Friday 23 May 2025, between 18:00 and 19:00: PSUR Repository (relevant for NCAs only).
For any further information, please contact EMA Service Desk
21-05-2025
Recommended use of web-based Human variations electronic Application
Forms (eAFs) for non-CAPs
Following the launch of optional use in February 2025, EMA now recommends
the use of
the PLM Portal web-based eAF
for all non-CAPs human variations, where possible.
This is aligned with the timeline and the roadmap towards mandatory use of the PLM Portal
web-based eAF for all human variations.
Please note that the interactive PDF eAF remains available for use
for all variation
procedures and while the PLM Portal eAF is now recommended for use for all procedure
types, there are some technical limitations, and in these cases you might need to use
the interactive pdf forms. Consult the guidance
and the release notes.
Please report any production issues through the
EMA ServiceDesk - Incident. Any change
request or other generic questions, recommendations, improvement suggestions please
raise them through EMA ServiceDesk - Request
PLM Portal web-based eAF use levels:
Optional Use: Both the interactive PDF and the web-based eAF are
available. Applicants
may choose either format, and no preference is expressed.
Recommended Use: The use of the web-based eAF is encouraged. However,
applicants may
continue to use the interactive PDF if they are not yet ready to adapt their internal
processes.
Strongly Recommended Use: The web-based eAF should be used in most
cases. The
interactive PDF should only be used if specific constraints prevent the use of the
web-based eAF (e.g., technical issues or missing features).
Mandatory Use: The use of the web-based eAF is required. Submissions
using the
interactive PDF will not be accepted. Mandatory use will only be introduced after a
formally announced transition period.
* Products authorised throughout mutual recognition procedure (MRP), decentralised
procedure (DCP) and national procedure (NAP)
14-05-2025
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates
to web eAF made in the version 1.2.0.4 released to production on 13 May 2025 are now
available on
PLM Portal and on the PLM Portal eAF web page.
Please note that a small number of products are affected by a synchronisation issue with
PMS and the forms where they are used cannot be exported and finalised. Once added the
products in the eAF, export the form and should you see the message
"Data refresh cannot be completed. Aborting the operation. Please retry again",
please use the interactive PDF instead. The teams are working on fixing this issue and
a new message will be published once it is resolved.
Additionally, the PLM portal FHIR XML version was updated to 2.2.2. The new version
introduces the following change: there is a new element in the Organisation, called
"alias", which contains (if existing) the selected alternative company name:
<alias value="alternative company/organisation name" />
The Release notes can be found here.
06-05-2025
eAF v1.27.0.1 (Human Variation, interactive PDF) now available, for use
from 1 May 2025
A new version of the interactive PDF Human Variation eAF v.27.0.1 was published today,
6th of May 2025, bringing a small change in the exported XML data. No other changes are
introduced. It is highly recommended to use this latest version of the form.
Reminder:
A new version of the Human Variation eAF v1.27.0.1 is now available
on the eAF website.
The version should be used starting with 1st of May 2025.
The version allows the
selection of Reference Member State and Concerned Member State(s) when the type of
authorisation is 'National Authorisation' and the type of
application is
'Super-grouping'.
It is mandatory to use version 1.27.0.1 for all new Human Variation
procedures. The version 1.27.0.0 for Human Variations will be removed from the eAF
website, however, users can continue to submit applications using this version for
ongoing procedures. Applicants are reminded that the version of the form should
not be
changed during an ongoing procedure.
29-04-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates
to web eAF made in the version 1.2.0.3 released to production on 28 April 2025 are now
available on
PLM Portal
and on the PLM Portal eAF web page.
Please note that a small number of products are affected by a synchronisation issue with
PMS and the forms where they are used cannot be exported and finalised. Once added the
products in the eAF, export the form and should you see the message "Data
refresh cannot
be completed. Aborting the operation. Please retry again", please use the
interactive
PDF instead. The teams are working on fixing this issue and a new message will be
published once it is resolved.
And
Planned maintenance of eSubmission systems on 30 April 2025, 18:00
CET
Due to planned maintenance, the following eSubmission systems will not be available on
Wednesday 30 April 2025, between 18:00 and 20:00: eSubmission website,
PSUR Repository,
Gateway Filehandler, Delivery File UI, Registration Gateway.
For any further information, please contact
EMA Service Desk.
28-04-2025
Reminder for PSUSA procedures: new requirement for Word version of the initial PSURs and RSI responses
When submitting initial PSUR or RSI responses for a PSUSA procedure,
the marketing authorisation holders (MAHs) are also required to systematically provide
Word versions of the submitted initial PSURs and RSI responses,
as part of the Working Documents folder.
When submitted with an eCTD, the Working Documents should always be provided in a
separate folder called "xxxx-workingdocuments" on the same submission zip package
containing the eCTD, where the number (xxxx) matches the number of the eCTD sequence being submitted.
Any deviation will lead to a failed submission, and the package will have to be
resubmitted with the correct naming. For example, if sending sequence "0007", which
contains working documents, the separate folder should be named "0007-workingdocuments".
More details can be found in chapter 2.9.10 of the
Harmonised guidance eCTD - version 6.0
25-04-2025
eAF v1.27.0.1 (Human Variation) now available, for use from 1 May
2025
A new version of the Human Variation eAF v1.27.0.1 is now available on
the eAF website. The version can be used starting with 1st of May 2025. The version allows the selection of
Reference Member State and Concerned Member State(s) when the type of authorisation is
'National Authorisation' and the type of application is 'Super-grouping'.
It is mandatory to use version 1.27.0.1 for all new Human Variation
procedures. The version 1.27.0.0 for Human Variations will be removed from the eAF
website, however, users can continue to submit applications using this version for
ongoing procedures. Applicants are reminded that the version of the form should
not be changed during an ongoing procedure.
16-04-2025
PSUSA procedures: new requirement for Word version of the PSURs and RSI responses
When submitting PSUR or RSI responses for a PSUSA procedure, the marketing authorisation holders (MAHs) are also required
to systematically provide Word versions of the submitted PSURs and RSI responses,
as part of the Working Documents folder.
Reminder
When submitted with an eCTD, the Working Documents should always be provided in a separate folder called "xxxx-workingdocuments" on the same submission zip package containing the eCTD, where the number (xxxx) matches the number of the eCTD sequence being submitted.
Any deviation will lead to a failed submission, and the package will have to be resubmitted with the correct naming. For example, if sending sequence "0007", which contains working documents, the separate folder should be named "0007-workingdocuments".
More details can be found in chapter 2.9.10 of the Harmonised guidance eCTD - version 6.0.
More details can be found in chapter 2.9.10 of the Harmonised guidance eCTD - version 6.0.
And
Planned maintenance of eSubmission systems on 23 April 2025, 18:00 CET
Due to planned maintenance, the Common Repository Web User Interface and API
(relevant for NCAs only) and the PSUR Repository Industry access and NCA access
will not be available on Wednesday 23 April 2025, between 18:00 and 20:00.
For any further information,
please contact EMA Service Desk.
04-04-2025
Update on web-based Human variations electronic Application Forms
(eAFs) timeline
EMA would like to provide you with an update on the progress of the web-based
Human
variations electronic Application Form (eAF) implementation on the
Product Lifecycle
Management (PLM) Portal.
During Q1 2025, we announced that the human variation web-based
eAF was open for first use for non-Centrally Authorised Products
(non-CAPs) through the
PLM Portal, allowing Marketing Authorisation Holders to use the
web-based application form for all EU variation procedures, including
submission to the NCAs.
Key points of the updated timeline:
Q2 2025:
- Recommended use of Human variation eAF for non-CAPs*.
- Continue the incremental release of the new UX design
and the incremental release of new features, including maintenance
and optimisation updates and performance improvements.
Q3 & Q4 2025:
- Strongly Recommended use of Human variation eAF for
non-CAPs*.
- Exploration of structured changes in eAF.
Please send any questions or concerns to
PLM.ValueStream@ema.europa.eu
*Exact date to be defined, on the condition that no major issues are identified
03-04-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates
to web eAF made in the version 1.2.0.2 released to production on 31 March 2025 are now
available on PLM Portal and on the PLM Portal eAF web page.
02-04-2025
eCTD 3.2.2 - validation criteria 8.1 to be updated
In the most recent validation criteria (version 8.1) for EU eCTD M1 specification, the rules 15.11 and 15.12 referring to the tracking table being mandatory for all submission types were updated. However, these rules do not apply to EDQM submissions; a new version of the EU eCTD M1 package will be published at a later stage.
In the meantime, for EDQM submissions, you can submit the packages even if the validation does not pass for rules 15.11 and 15.12.
28-03-2025
Planned maintenance of eSubmission systems on 31 March 2025, 18:00 CET
Due to planned maintenance and the activation of the Common Repository API MFA, the Common Repository Web User Interface and API (relevant for NCAs only) will not be available on Monday 31 March 2025, between 18:00 and 22:00.
For any further information, please contact EMA Service Desk.
25-03-2025
Updated PLM Portal eAF guide to navigation now available
An updated version of the PLM Portal eAF guide to navigation is now available.
19-03-2025
Planned maintenance of eSubmission systems on 24 March 2025, 18:00 CET
Due to planned maintenance, the Common Repository Web User Interface and API (relevant for NCAs only) and PSUR Repository Web User Interface and API (relevant for NCAs only) will not be available on Monday 24 March 2025, between 18:00 and 22:00
For any further information, please contact EMA Service Desk.
19-03-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates
to web eAF made in the version 1.2.0.1 released to production on 17 March 2025 are now
available on PLM Portal and on the PLM Portal eAF web page.
14-03-2025
Planned maintenance of eSubmission systems on 17 March 2025, 18:00 CET
Due to planned maintenance, the Common Repository Web User Interface and API (relevant for NCAs only) and PSUR Repository Web User Interface and API (relevant for NCAs only) will not be available on Monday 17 March 2025, between 18:00 and 22:00
For any further information, please contact EMA Service Desk.
07-03-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates to web eAF made in the version 1.2.0.0 released to production on 06 March 2025 are now available on
PLM Portal
and on the PLM Portal eAF web page.
And
Planned maintenance of PSUR Repository API on 7 March 2025, 18:00 CET
Due to planned maintenance, the PSUR Repository API (relevant for NCAs only) will not be available on Friday 7 March 2025, between 18:00 and 19:00.
For any further information, please contact
EMA Service Desk.
05-03-2025
PLM Portal eAF – Integrity stamp go-live on 6 March
EMA is pleased to announce that the PLM Portal eAF Integrity stamp feature will be deployed Thursday, 6th of March, after 18:00 CET.
The main impact and changes are as follows:
- Upon 'Finalisation', the PLM Portal web based human variation eAF will be locked and moved to the 'Completed' tab (the form can be opened for further editing if necessary)
- The form will be locked with a digital 'signature' called eAF integrity warranty (or eAF integrity stamp)
- Will be launched on 6th March 2025 – after this date the system will include the stamp on all forms that are finalised
- Forms that have not been finalised (i.e. do not contain the integrity stamp) will be rejected by the regulators in the future – date to be confirmed!
- The applicants will still be able to include additional digital signature(s) into the finalised form (exported pdf)
- It will not be possible to include an image of a signature or an 'adobe signature' to the form after the stamp is included
- There is no integrity stamp in the ‘legacy’ interactive pdf eAFs
If you have any question please contact plm.valuestream@ema.europa.eu or raise a request in the EMA Service Desk..
AND
PLM Portal FHIR XML version upgrade
The PLM portal FHIR XML version was updated to 2.2.1. The new version introduces the following change: the <meta> node will contain a new profile value (<profile value=http://ema.europa.eu/fhir/definition/fhir-export/2.2.1.0 />) and the "Bundle-variation" will lose the 4th digit in the XML.
The Release notes can be found here.
26-02-2025
Planned maintenance of eSubmission systems on 4 March 2025, 18:00 CET
Due to planned maintenance, improvements in the Delivery File User Interface and for the Multi Factor Authentication go-live in Common Repository (relevant for NCAs only) and PSUR Repository (relevant for NCAs only), the following eSubmission systems will not be available on Tuesday 4 March 2025, between 18:00 and 22:00: Common Repository (Web User Interface and API), PSUR Repository (Web User Interface and API), Gateway Filehandler, Delivery File User Interface.
For any further information, please contact
EMA Service Desk.
And
Planned maintenance of Common Repository on 27 January 2025, 18:00 CET
Due to planned maintenance and in preparation to the MFA activation, the following eSubmission systems will not be available on Thursday 27 February 2025, between 18:00 and 22:00: Common Repository Web User Interface and Common Repository API (both applicable for NCAs only).
For any further information, please contact
EMA Service Desk.
And
Planned maintenance of PSUR Repository (Industry access) on 28 January 2025, 18:00 CET
Due to planned maintenance, the PSUR Repository (Industry access) will not be available on Friday 28 February 2025, between 18:00 and 20:00.
For any further information, please contact
EMA Service Desk.
20-02-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release Notes
reflecting bug fixes and updates to web eAF made in the version 1.1.1.4 released to
production on 18 February 2025 are now available on
PLM Portal
and on the PLM Portal eAF web page.
18-02-2025
ESMP: full scope of ESMP functionalities live on 29 January
As of 29 January 2025, the
European Shortages Monitoring Platform (ESMP) is fully
operational
. In addition to the routine shortage reporting by marketing authorisation
holders (MAHs) of centrally authorised products (CAPs), launched in November 2024, the
full scope of functionalities of the ESMP is available for crisis
and MSSG-led
preparedness reporting, for both MAHs and national competent authorities
(NCAs). These
newly released functionalities will be active in ESMP in crises and preparedness
situations for affected MAHs of CAPs and non-CAPs according to the list of medicines
published for the specific situations.
The ESMP will enable information exchange for prevention, identification and management
of shortages to ensure medicines are available for patients in the EU and EEA.
A dedicated training session for all MAHs will be held on 19
February 2025 from
10:00-12:30 CET. Registration is available
here (MAH training registration - WebEx) and
more information is available on the training event page on EMA's corporate website. The
session will be live broadcast and a recording will be published on the event page.
Further, EMA organises a Q&A session for CAP MAHs on the routine CAP
shortage reporting
functionalities on 25 February 2025, 10:00 - 11:00 CET. Find more
information
here on
the Q&A clinic (event page) and registration is available via this link (Q&A for CAP
MAHs - WebEx). The session will be recorded, and the recording will be made
available on
the event page.
Finally, please note that all relevant and continuously updated ESMP materials can be
found on the
ESMP webpage on the EMA website.
13-02-2025
Production go-live for submissions of human variations web-based eAFs for non-CAPs
As previously announced through our
Product Lifecycle Management (PLM) newsletter
(27/01/2025) the EMA is pleased to confirm as of Tuesday 11 February 2025, the human variations web-based electronic Application Form (eAF) is open for first use for non-Centrally Authorised Products* (non-CAPs) within the
PLM Portal.
Please note that the
interactive PDF eAF
remains available for use for all variation procedures and while the PLM Portal eAF is now available
for use for all procedure types, there are some technical limitations, and in these cases the applicants are requested to use the interactive pdf forms.
* Products authorised throughout mutual recognition procedure (MRP), decentralised procedure (DCP) and national procedure (NAP)
11-02-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates to web eAF made in the version 1.1.1.3 released to production on 3 February 2025 are now available on
PLM Portal
and on the PLM Portal eAF web page.
And
PLM Portal FHIR XML version upgrade
The PLM portal FHIR XML version was updated to 2.2.0, however no change affecting the web-based eAF XML was introduced. The Release notes can be found
here.
05-02-2025
Planned maintenance of the Common Repository on 5 February 2025
Due to planned maintenance, the Common Repository (User interface and API) will not be available on Wednesday 5 February 2025, between 18:00 and 20:00. For any further information, please contact EMA Service Desk.
03-02-2025
PMS User Interface edit functionalities now live for Industry users!
The Product Management Service (PMS) User Interface (PUI) edit functionalities - live on the
Product Lifecycle Management (PLM) portal
- are now available for Industry users.
Note that the write access is for now limited to pack size and manufacturer data for non-Centrally Authorised Products* (non-CAPs).
This data is indeed required for the shortage reporting via the
European Shortages Monitoring Platform (ESMP), which went live with full functionalities on 29 January 2025.
This milestone builds on the previous go-live of the
PMS Application Programming Interface (API) in read-only mode
(for Marketing Authorisation Holders (MAHs) on 3 July 2024, for H&V National Competent Authorities (NCAs) in September 2024 and for all NCAs in December 2024) and the launch of the
PUI in read-only mode for CAPs and non-CAPs
(respectively on 31 May 2024 and 19 September 2024).
Please consult this news article
for full PMS roadmap and a recap of key current and future actions for MAHs.
Supporting events:
- Training webinar: EMA held a public webinar on 28 January 2025 to showcase PMS released PUI edit functionalities (enrichment process). Consult the
event web page to access presentation and recording.
- Weekly Q&A clinics on PUI & API in February:
PUI guidance documents:
* Products authorised throughout mutual recognition procedure (MRP), decentralised procedure (DCP) and national procedure (NAP)
29-01-2025
Updated eAF 1.27.0.0 Human Variation form
The Human Variation form v1.27.0.0 was updated on the 29 January 2025. The change
follows the recent update of the new variation regulation; in the Declaration section in
the parallel procedures sub-section, the table with the product/procedure details has
been removed.
It is recommended to use this latest form for new submissions. Please
note that there is no version number change and that the release notes will be updated
and published in the relevant section of the eAF page.
21-01-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates
to web eAF made in the version 1.1.1.2 released to production on 20 January 2025 are now
available on PLM Portal and on the PLM Portal eAF web page.
AND
eCTD 3.2.2 - validation criteria 8.1 to be updated
In the most recent validation criteria (version 8.1) for EU eCTD M1
specification, the
rules 15.11 and 15.12 referring to the tracking table
being mandatory for all submission
types were updated. However, these rules do not apply to EDQM submissions, and therefore
a further communication and potential update of the EU eCTD M1 package will be
published. In the meantime, for EDQM submissions, you can use the previous validation
criteria package (7.1).
14-01-2025
Updated version of the "EU eCTD v4.0 Controlled Vocabularies" (.xml format)
An updated version of the EU eCTD v4.0 Controlled Vocabularies is now available
here.
The updated package contains the missing list (territorial authority) and the unused list was removed (dosage form category).
13-01-2025
Planned maintenance of eSubmission systems on 14 January 2025, 18:00 CET
Due to planned maintenance, the following eSubmission systems will not be available on Tuesday 14 January 2025, between 18:00 and 19:30: Gateway XML delivery file user interface, Gateway Filehandler, PSUR Repository Web-UI.
For any further information, please contact EMA service desk.
|

