|
|
 |
|
09-01-2025
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates to web eAF made in the version 1.1.1.1 released to production
on 7 January 2025 are now available on
PLM Portal
and on the PLM Portal eAF web page.
Important note to PLM Portal users:
Due to an identified issue with ingredient data on the PLM Portal, the following medicinal product data will be removed from the Product Lifecycle Management Portal from 13 to 17 January 2025:
- All ingredients and their strengths
- Pharmaceutical products and routes of administration
- Package Items, Package Item Materials, Package Manufactured Items
- ATC codes
This means the above data will be unavailable in Product Management Service Product User Interface and Human Variations electronic Application Form.
In practice, for PLM Portal web-based eAF users this means that fields containing this information will be empty in the eAFs generated from the PLM Portal during this period. The missing data is not expected to impact the users of the eAF for variations for Centrally Authorised Products; and eAFs submitted with empty fields where the data is ‘missing’ due to the data cleansing exercise will not be rejected. We kindly request you not to report missing data/data issues in the eAF via the EMA Service desk during this period.
This means that PLM Portal eAF users should be able to continue to use the web-based form during this period unless unexpected issues, that were not found during testing, are found once the data cleansing process starts. However, users should note that the interactive pdf eAF is available for use for all variation procedures and applicants may feel more comfortable using the
pdf eAF
.
From 20 January 2025, the removed data will be re-uploaded in phases:
- All Centrally Authorised Products' (CAPs) data will be available from 20 January 2025;
- Non-CAPs data will gradually reappear over the following two weeks.
08-01-2025
ESMP pre-launch for MAHs and available training materials
As of 28 November 2024, the
European Shortages Monitoring Platform (ESMP)
is officially live for routine shortage reporting by marketing authorisation holders (MAHs).
Marketing authorisation holders (MAHs) can now submit data to routinely report shortages of centrally authorised medicines (CAPs) to EMA. This marks the start of a transition period that will end on 2 February 2025, when the use of the platform becomes mandatory for CAP shortage reporting to EMA. National reporting requirements remain applicable. The ESMP will enable information exchange for prevention, identification and management of shortages to ensure medicines are available for patients in the EU and EEA.
The full first version of the ESMP will be released by 2 February 2025 and will expand the platform's functionalities to include supply, demand, and availability reporting during crises and MSSG-led preparedness exercises by MAHs and NCAs.
Further information, including training materials, user guidance, and
ESMP webinar
and
ESMP training
event recordings, are available on EMA's website to support stakeholders. For more details, visit the
ESMP webpage
and the
press release on EMA's website.
13-12-2024
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates to web eAF made in the version 1.1.1.0 released to production
on 9 December 2024 are now available on
PLM Portal
and on the PLM Portal eAF web page.
And
Update on the start of strongly recommended use of web-based eAF for NCA submissions
The expected start date of the strongly recommended use of the PLM Portal web-based eAF
submissions to the National Competent Authorities for non-CAPs variations is now planned
for February 2025. This updated timeframe is based on the latest projections by EMA’s development teams.
Please note that the web-based eAF is fully functional for the submission of variations
for CAPs, including EMA-led worksharing variations containing non-CAPs.
Should you have any questions or require further information, please do not hesitate to contact us at
plm.valuestream@ema.europa.eu.
12-12-2024
EU eCTD v4.0 Controlled Vocabularies in .xml format now available
The package containing genericode (.xml) format for the first version of the EU
eCTD v4.0 Controlled Vocabularies is now available
here.
They are complementing the .xlsx version which was published in October.
12-12-2024
Updated PLM Portal eAF FHIR XML Release notes now available
An updated version of the
PLM Portal eAF FHIR XML Release notes
reflecting the implementation of FHIR XML
2.1.1 in the PLM Portal eAF (released to
production on 9 December 2024)
are now available on the
PLM Portal
and in the documentation section of the
PLM Portal eAF, together with several updated documents to
provide consumers of the FHIR XML message the easiest
possible way to upgrade to the eAF XML 2.1.1 or to
start
the implementation now with the latest version.
10-12-2024
eSubmissions Gateway – naming of the working documents folder
When submitted with an eCTD, the Working Documents should always be provided in a separate folder called "xxxx-workingdocuments" on the same submission zip package containing the eCTD, where the number (xxxx) matches the number of the eCTD sequence being submitted.
Any deviation will lead to a failed submission, and the package will have to be resubmitted with the correct naming. For example, if sending sequence "0007", which contains working documents, the separate folder should be named "0007-workingdocuments".
More details can be found in chapter 2.9.10 of the Harmonised guidance eCTD - version 6.0.
05-12-2024
EU Harmonised technical eCTD guidance version 6.0 now available
A new updated version of the eCTD EU Harmonised technical guidance is now available
here, together with the Release notes.
The EU Harmonised technical guidance is aligned with the EU M1 eCTD Specification v3.1
and the Validation Criteria v8.1.
The timeline for implementation is as follows:
- (optional use): From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and validation criteria v7.1 or v8.1 are accepted.
- (mandatory use): From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.1 are accepted.
And
Updated version of the VNeeS specification v3.2 now available
The guideline on eSubmissions for Veterinary products version 3.2 has now
been published. It will enter into force on 1 January 2025 (see links
under section "Current Guidance" below).
The update is related to text changes only (detailed in the release notes for
version 3.2) and it does not affect the validation criteria.
And
Updated VNeeS Q&A relating to eSubmission for Veterinary Applications now available
The VNeeS Q&A relating to eSubmission for Veterinary Applications was updated and it can be found in the Current Guidance section.
04-12-2024
Planned maintenance of eSubmission systems on 5 December 2024, 18:00
CET
Due to planned maintenance, the following eSubmission systems will not be available on
Thursday 5
December 2024,
between 18:00 and 24:00: Gateway XML delivery file user interface, Gateway Filehandler,
Common
Repository Web-UI
and API, PSUR Repository Web-UI (both NCA and industry access) and API.
The maintenance includes various improvements and implementations:
- Common Repository Web-UI - technology modernisation
- Gateway XML delivery file user interface - various improvements and integrations for
retrieval of data (e.g. procedures) from the internal EMA case management system (IRIS)
- Gateway XML delivery file user interface - minor changes related to the new variation regulation (e.g.
renaming of the submission mode from "IG" to "super-grouping")
Updated documentation will be published on the relevant pages of the
eSubmission website.
For any further information, please contact EMA Service Desk.
03-12-2024
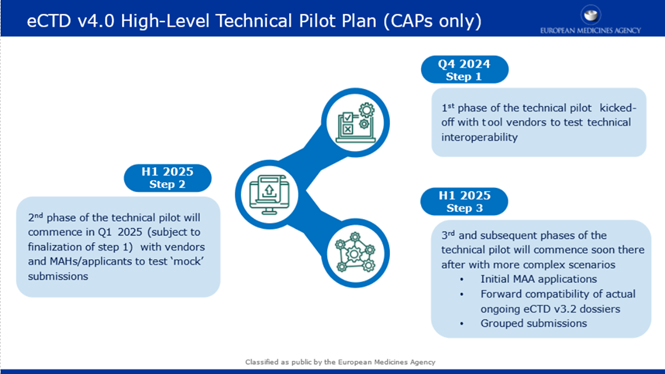
Call for Interest eCTD v4.0 Technical Pilot
The electronic Common Technical Document (eCTD) v4.0 is pleased to announce the launch
of the eCTD v4.0
Technical Pilot and invite interested eCTD tool vendors to participate in Step
1. A call for participation in
the following steps will be launched at a later stage.
If you have not yet expressed your interest, please send an email to eCTD4consultation@ema.europa.eu by 20
December 2024 to express your intention to participate. If approved, you will
receive a confirmation e-mail with further instructions.
Step 1
- Participants: Tool vendors, EMA
- Timeline: Q4 2024 - Q1 2025
- Focus Area: Technical Interoperability
Step 2
- Participants: Tool vendors (potential collaboration with MAHs), EMA
- Timeline: Q1 2025 (Subject to finalisation of step 1) - TBD
- Focus Areas:
- Simple scenarios (mock submissions, single package/sequence)
- Document lifecycle management (e.g. replacements, updates)
- Handling of multiple file formats, pack sizes, manufacturers
- Review of controlled vocabularies (CV)
- Updating keywords, priority numbers, and document titles
- Document reuse
Step 3
- Participants: Tool vendors, MAHs, EMA
- Timeline: Subject to finalisation of step 2, TBD
- Focus Areas:
- Expansion of Step 2 scenarios with non-mock data
- Grouped submissions functionality, forward compatibility
- Parallel regulatory activities
PFor a high-level view, please refer to the attached pilot plan.
02-12-2024
Updated eAF v1.27.0.0 (Human Variation) now available, for use from 1
January 2025
An updated/corrected version of the eAF 1.27.0.0 (Human variation) is now available on
the eSubmission website.
The difference compared to the version published on the 27th of November is
in the exported XML, which now
correctly contains the super-grouping checkbox.
It is mandatory to use version 1.27.0.0 for all Human Variation applications with
procedure start date after 1st of January 2025.
The version 1.26.0.0 for Human Variations will be removed from the eAF website, however,
users can continue to
submit applications using this version for procedures starting until 31 December 2024.
Applicants are reminded
that the version of the form should not be changed during an ongoing
procedure. Please note that in case you
need to provide an update to a form that has been submitted prior to 1st of
January 2025, you should use the
previous version (1.26.0.0).
27-11-2024
EMA encourages companies to submit type I variations for 2024 by end
November 2024
EMA is advising marketing authorisation holders to submit type IA and type
IAIN variations for 2024 no later than 30 November 2024.
This will enable EMA to acknowledge the validity of the submissions before the Agency's
closure between 20 December 2024 and 5 January 2025,
within the 30-day timeframe set out in Article 14 of
Commission Regulation (EC) No 1234/2008.
Marketing authorisation holders are advised to submit any type IB variations or
groupings of type IBs and type IAs by 30 November 2024 for a start of procedure
in 2024. For submissions received on or after 1 December 2024, the procedure may not
start until January 2025.
and
eAF v1.27.0.0 (Human Variation) now available, for use from 1 January
2025
In line with the amended Variation Regulation entering into force on the 1st
of January 2025 (
Guidance on the application of the amended Variations Regulation from 1 January 2025
| European Medicines Agency (EMA)
), a new version of the Human Variation eAF v1.27.0.0 is now available
on the eAF website.
It is mandatory to use version 1.27.0.0 for all Human Variation applications with
procedure start date after 1st of January 2025.
The version 1.26.0.0 for Human Variations will be removed from the eAF website, however,
users can continue to submit applications using this version for procedures
starting until 31 December 2024. Applicants are reminded that the version of the form
should not be changed during an ongoing procedure.
Please note that in case you need to provide an update to a form that has been submitted
prior to 1st of January 2025, you should use the previous version (1.26.0.0).
The form, the release notes and the associated files (i.e. schema and dictionary) are
published in the relevant sections of the eAF webpage.
26-11-2024
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates to web eAF made in the version 1.1.0.3 released to
production
on 25 November 2024 are now available on
PLM Portal
and on the PLM Portal eAF web page.
18-11-2024
Updated PLM Portal eAF Release notes now available
An updated version of the
PLM Portal eAF Release notes
reflecting bug fixes and updates to web eAF made in the version 1.1.0.2 released to
production
on 11 November 2024 are now available on
PLM Portal
and on the PLM Portal eAF web page.
30-10-2024
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes
reflecting bug fixes and updates to web eAF made in the version 1.1.0.1 released to
production on 28 October 2024 are now available on PLM Portal and on the PLM Portal eAF web page.
29-10-2024
Interactive PDF eAF technical documentation updated
An updated set of schemas were published under the "Technical Documents"
section of eAF (interactive PDF), relevant for small changes in the XML schema
definition for versions 1.26.0.0 and 1.26.0.1. The changes are reflected in the "eAF DES
change summary" document.
28-10-2024
Minor update to the published EU Validation criteria
An updated version of the validation
criteria to add further clarification the has been published on the eSubmission website. The version is
related to the EU Module 1 Specification version 3.1 and should be used in case of
submitting a new sequence according to EU M1 specification v3.1. The new validation
criteria will be used for the technical validation for all v3.1 electronic submissions
received as of 1 March 2025 to the NCAs and EMA. The changes are
reflected in the Release Notes.
From 1 December 2024 eCTDs compliant with EU M1 v3.0.4
or v3.1 and validation criteria v7.1, 8.0 or v8.1 are
accepted.
From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation
criteria v8.1 are accepted.
24-10-2024
Planned maintenance of eSubmission systems on 24 October 2024, 18:00
CET
Due to planned maintenance, the following eSubmission systems will not be
available on Thursday 23 October 2024, between 18:00 and 20:00: Gateway XML delivery
file user interface, Gateway Filehandler and PSUR Repository XML delivery file UI
(relevant for industry only).
Previously created delivery files will continue to work. For any further information,
please contact EMA Service
Desk.
14-10-2024
Non-Centrally Authorised Products now available in the PLM Portal
eAF
The EMA is pleased to announce that the non-Centrally Authorised
Products* (non-CAPs) data are now available in the Human variation
web-based eAF within the Product Lifecycle Management (PLM)
Portal.
Please note that, due to system limitations and pending future improvements, the
web-based eAF cannot currently be used for submissions to the National Competent
Authorities. However, the web eAF is fully functional for the submission of
variations of CAPs, including EMA-led worksharing variations containing CAPs and
non-CAPs.
Please join the training session on 17 October 2024 10:00 - 11:30
(CEST).
Please see an updated version of the draft PLM Portal eAF guide to navigation is now available.
04-10-2024
EU eCTD v4.0 draft Implementation package now available
An updated draft version of the EU eCTD v4.0 implementation package is now
available here.
The package contains the following:
- EU eCTD v4.0 Implementation Guide draft version 1.2 ; this version is focused on CAPs and the main changes since version 1.1 are reflected in the "Document change history" of the file
- EU eCTD v4.0 Controlled Vocabularies, in .xlsx format (version 1); the genericode files (.xml format) will be published in the following weeks
- EU eCTD v4.0 Accepted file formats
An upcoming updated package (containing the genericode files for the controlled vocabularies and the validation criteria) will be made available in the coming period.
The package is meant for eCTD tool vendors to access the new format of the Controlled Vocabularies and the new OIDs, as well as to be notified by the changes in the EU eCTD v4.0 Implementation Guide (version 1.2).
According to the EU eCTD v4.0 implementation timeline, a technical pilot for eCTD v4.0 in EU (focused on CAPs) is planned to start by the end of 2024, and more details will be published on the eSubmission website. If you are an eCTD tool vendor, have already developed eCTD v4.0 capabilities in the tool and are interested in participating in the EU eCTD v4.0 pilot, follow the eSubmission website for the upcoming announcement, or send your intention to join the pilot at ectd4consultation@ema.europa.eu.
03-10-2024
Non-CAPs data available in the PLM Portal web based eAF from 14th October 2024
The EMA is pleased to announce that, as of the 14 October 2024, non-Centrally
Authorised Products* (non-CAPs) data will be available in the Human variation
web-based eAF within the Product
Lifecycle Management (PLM) Portal.
Please note that, due to system limitations and pending future improvements, the web-based eAF
cannot currently be used for submissions to the National Competent Authorities. However,
the web eAF is fully functional for the submission of variations of CAPs, including EMA-led
worksharing variations of non-CAPs.
In order to familiarise with the new functionality of web-based eAFs for Human non-CAP
variation procedures, the eAF team is providing a dedicated training session on
17 October 2024 10:00 - 11:30 (CEST). During this event, access and navigation of
web-based eAF for non-CAPs will be showcased. We would highly recommend all relevant Industry
stakeholders to participate to this training: Register here
Key actions for Marketing Authorisation Holders:
- Register as an eAF user in the PLM Portal following the registration process and log into the eAF for familiarisation and testing purposes;
- Join upcoming training session;
- Follow EMA announcements to be informed of the date when web-based eAF can be submitted to the NCAs. This is currently expected to start in January 2025;
- Continue to submit the interactive PDF eAFs to the NCAs for non-EMA led procedures;
- Gather user feedback related to non-CAP variations within your organisations (MAHs and industry organisations) and to provide this consolidated feedback to EMA via plm.valuestream@ema.europa.eu.
Key actions for NCAs to:
- Follow EMA announcements to be informed of the date when they will start receiving web-based eAF submissions.
- Forward any non-CAP variations related user feedback from MAHs to EMA via plm.valuestream@ema.europa.eu
- Join upcoming information session on the go-live of the web-based eAF for non-CAPs - to take place in November 2024
.
eAF guidance documents available to support your work on the PLM:
- Guide for PLM Portal Registration - Document
- Guide for PLM Portal eAF Navigation - Document
Please be reminded that:
- External testing of the eAF User Acceptance Testing (UAT) is not foreseen for 2024
- No transition towards mandatory use of the Human variations web-based eAF will start before a UAT takes place
If you have any question please contact plm.valuestream@ema.europa.eu
or post them via the PLM
Portal Forum.
* Products authorised throughout mutual recognition procedure (MRP), decentralised procedure (DCP)
and national procedure (NAP)
01-10-2024
Planned maintenance of eSubmission systems on 8 October 2024, 18:00 CET
Due to planned maintenance, the following eSubmission systems will not be available on
Tuesday 8 October 2024, between 18:00 and 20:00: Gateway XML delivery file user interface, Gateway
Filehandler and PSUR Repository Web-UI (relevant for NCAs only).
The maintenance includes the aligning of the VNeeS submission format to 3.1, where applicable, and
other small improvements.
Previously created delivery files will not continue to work, please use the new version of the Gateway
XML delivery file after the maintenance has completed.
Updated documentation will be published on the relevant pages of the eSubmission website. For any
further information, please contact EMA
Service Desk.
20-09-2024
Updated PLM Portal eAF guide to navigation now available
An updated version of the draft PLM Portal eAF guide to navigation
is now available.
And
Updated PLM Portal eAF Release notes now available
An updated version of the PLM
Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.19
released to production on 18 September 2024 are now available on PLM
Portal and on the PLM Portal eAF web page.
19-09-2024
Completion of non-CAPs data load on PMS Product UI
The EMA is pleased to inform you that the data load of non-Centrally Authorised
Product* (non-CAP) information on the PMS Product User Interface (PUI) has been successfully
completed. As of now, all human non-CAP and CAP data is available in read-only mode via the
PUI on the Product Lifecycle Management
(PLM) Portal.
National Competent Authorities and Marketing Authorisation Holders can now request the new PMS roles
needed for accessing the PMS PUI through the EMA Account
Management portal. A training session is scheduled for 16 October
2024 (10:00 - 11:00, CEST), where the PMS team will provide an overview of the PUI,
showcase the access and navigation and answer your questions. Registration is available here. Meanwhile, you can consult the presentation & recording of the previous training session on PUI, held on
3 June 2024, and the PMS PUI guidance documents.
Please be reminded that this data load will not yet trigger the availability of non-CAPs data
in the web-based eAF. Once performance improvements are completed, non-CAPs data will
become available in the eAF.
Read access to both CAPs and non-CAPs through the PMS PUI is a key step toward the
data-centric target operating model. This milestone builds on the go-live
of the PMS Application Programming Interface (API) for CAPs and non-CAPs in read-only mode for
MAHs on 3 July 2024 and the launch
of the PMS PUI in read-only mode for CAPs on 31 May 2024.
* Products authorised throughout mutual recognition procedure (MRP), decentralised procedure (DCP) and
national procedure (NAP)
11-09-2024
Start of non-CAPs data load on PMS Product UI
The EMA is pleased to inform you that non-Centrally Authorised Products* (non-CAP)
data are currently being loaded in read-only mode on the Product Management Service (PMS) Product User
Interface (PUI), live on the live on the Product Lifecycle Management
(PLM) Portal. This data load is expected to be completed by the end of September 2024, after which
all non-CAP data will be available in read-only mode on the PUI.
IMPORTANT: To avoid platform overload and preserve the performance of the PLM
platform while the data load is ongoing, the EMA requests that National Competent Authorities
(NCAs) refrain from requesting PMS roles at this time. Current PLM PUI and eAF
users are also strongly encouraged to minimise activity on the PLM Portal.
Please note, this data load will not yet trigger the availability of non-CAPs in the web-based
electronic Application Form (eAF). Following the deployment of further performance
improvements, non-CAPs will become available also in the eAF. During the non-CAP data load, users
of the PLM Portal and IRIS Industry & Network Portal may experience minor performance
issues, such as slower system responses. In such circumstances, users do not need to
raise this via EMA Service Desk.
* Products authorised throughout mutual recognition procedure (MRP), decentralised procedure (DCP) and
national procedure (NAP)
06-09-2024
Updated PLM Portal eAF Release notes now available
An updated version of the PLM
Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.18
released to production on 06 September 2024 are now available on PLM
Portal and on the PLM Portal eAF web page.
28-08-2024
Updated PLM Portal eAF guide to navigation now available
An updated version of the draft PLM Portal eAF guide to navigation
is now available. The change is in chapter 2.2.4 (Add product), page 40, and it is related to the
introduction of a temporary popup message, while the adding of the products and all the afferent
processes are finalised.
And
Updated PLM Portal eAF Release notes now available
An updated version of the PLM
Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.17
released to production on 27 August 2024 are now available on PLM
Portal and on the PLM Portal eAF web page.
27-08-2024
Planned maintenance of the eSubmission Gateway XML delivery file on 2 September
2024
Due to planned maintenance, the eSubmission Gateway XML delivery file will not be available
on Monday 2 September 2024, between 18:00 and 19:00. For any further information, please contact EMA Service Desk.
08-08-2024
Updated PLM Portal eAF Release notes now available
An updated version of the PLM
Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.16
released to production on 08 August 2024 are now available on PLM
Portal and on the PLM Portal eAF web page.
23-07-2024
Updated PLM Portal eAF Release notes now available
An updated version of the PLM
Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.15
released to production on 22 July 2024 are now available on PLM Portal Forum
and on the PLM Portal eAF web page.
22-07-2024
Updated PLM Portal eAF guide to navigation now available
An updated version of the draft PLM Portal eAF guide to navigation
is now available.
17-07-2024
Updated PLM Portal eAF Release notes now available
An updated version of the PLM
Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.14
released to production on 15 July 2024 are now available on PLM Portal Forum
and on the PLM Portal eAF web page.
11-07-2024
Invitation to register for the Info Session on PLM Portal eAF - Add package
EMA will host an Information session on 18 July 2024, 11:00 - 12:00 (CEST)
to explain and showcase the latest developments and bug fixes deployed in the PLM Portal web-based
electronic Application Form (eAF), namely the add package feature. Participants will
have the opportunity to ask questions in the last part of the session.
The eAF add package functionality allows the applicants to use the web based eAF for variations adding
a new pack size.
The participation is recommended to Industry stakeholders who are filling in application forms for
their respective organisations.
Register here, and check the event web page for agenda, presentation and recording.
01-07-2024
Updated PLM Portal eAF FHIR XML Release notes now available
An updated version of the PLM Portal eAF FHIR XML Release notes
reflecting the implementation of FHIR XML 2.0.1 in the PLM Portal eAF (released to
production on 28 June 2024) are now available on the PLM
Portal and in the section of the PLM Portal eAF,
Documentation section, together with several updated documents to provide consumers of the FHIR XML
message the easiest possible way to upgrade to the eAF XML 2.0.1 or to start the implementation now
with the latest version.
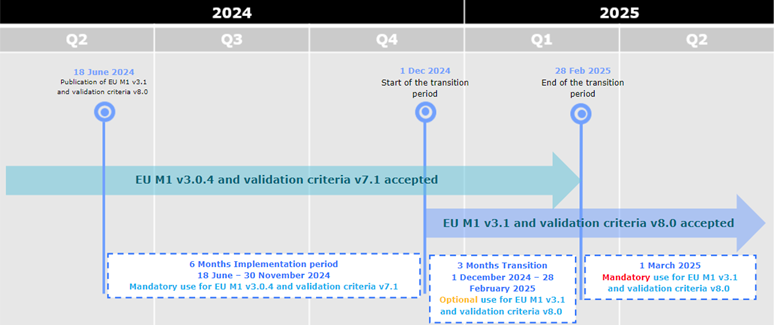
18-06-2024
eCTD 3.2.2 new package available
A new version of the EU eCTD M1
Specification, version 3.1, is now published on the eSubmission
website
The version 3.1 sees the introduction of a number of changes across the specification, however, more
specifically, the introduction of a new annex detailing the list of accepted
file formats
(Reminder: the generally accepted file format is the PDF, however, in specific cases, other formats
will be exceptionally accepted in the eCTD modules). The changes are reflected in the Release
Notes.
The same changes are reflected in the additional files of the EU M1 Implementation Guide package
(i.e. eu-envelope.mod, eu-regional.dtd, eu-regional.xsl)
A new version of the validation criteria has been published on the eSubmission website. The version is related to the
EU Module 1 Specification version 3.1 and should be used in case of submitting a new sequence
according to EU M1 specification v3.1. The new validation criteria will be used for the technical
validation for all v3.1 electronic submissions received as of 1 March 2025 to the
NCAs and EMA. The changes are reflected in the Release Notes.
During the initial period of 6 months from 18 June 2024 to 30 November 2024,
applicants can only submit eCTD format submissions compliant with EU M1 v3.0.4 and
validation criteria version 7.1.
From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and
validation criteria v7.1 or v8.0 are accepted.
From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.0
are accepted.
13-06-2024
ICH Call for Vendor participation now open
A call for vendor participation is now published on the ICH Official website.
If you are an eCTD v4.0 Tool Vendor and would like to join the eCTD Tool Vendor Group to discuss eCTD
v4.0 implementation, please send the following information listed below to ICHM8Vendors@ich.org:
- First Name;
- Last Name;
- Email;
- Company Name;
Please note that your information will be shared as needed with Representatives of ICH
Members and Observers involved with ICH eCTD activities.
The Vendor Group kick-off meeting will be held on 17 July 2024, at 7.00 A.M. ET.
The vendor group is solely intended to facilitate collaborative discussion on the eCTD v4.0
Implementation Specification and timelines.
More information can be found on the ICH Official web site: ICH.
AND
ICH eCTD v4.0 package updated
The ICH eCTD v4.0 package has been updated in response to the change requests and/or
discussion within the EWG after its initial release. There has been number of updates to the ICH IG
and both the Support Documentation and the Orientation Material presentations have been updated.
Additionally, as of May 2024, the Implementation Guide and Controlled Vocabulary documents were split into two different packages to
enable Controlled Vocabulary Versioning.
More information can be found on the ICH Official web site: ICH.
11-06-2024
Planned maintenance of the PSUR Repository (NCAs only) on 18 June 2024, 18:00
CET
Due to planned maintenance, the PSUR Repository NCA UI and PSUR Repository NCA API will not
be available for approximately 1 hour on Tuesday, 18 June 2024, after 18:00 CET. The maintenance is
related to a technical upgrade and will not result in any changes of the features and functionalities
of the system.
AND
Planned maintenance of the eSubmission systems on 19 June 2024, 18:00 CET
Due to planned maintenance, the following eSubmission systems will not be available on
Wednesday, 19 June 2024, between 18:00 and 21:00: Common Repository Web-UI (relevant for NCAs
only) and Common Repository API (relevant for NCAs only). During the same interval, the
Gateway Filehandler will experience short delays in sending the acknowledgements of the processed
submissions.
03-06-2024
PMS Product UI Now Live on the PLM Portal
The PMS Product User Interface (PUI) was launched on 31 May 2024, in read-only mode on Product Lifecycle Management (PLM)
Portal. Registered users are now able to view Centrally Authorised Product - (CAP) data in the PUI
(Nationally Authorised Product (NAP) data will be available in early Q3 2024).
Please note that the eAF users have now automatically access to the PUI. Consult these guidance
documents and join the 3 June 2024 training session to prepare for registration & navigation of PUI.
17-05-2024
Updated PLM Portal Guide to Navigation is now available
Please find an updated version of the draft PLM Portal eAF Guide to Navigation here.
AND
New Paediatric submissions are mandatory via IRIS platform from 4 June 2024
Please note that from 4 June 2024, the following types of new
paediatric submissions must be carried out via IRIS:
- Initial paediatric investigation plan (PIP)
- Modification of an agreed PIP
- Product-specific waiver
- Compliance check
- Annual report on paediatric deferred measures
- Confirmation of applicability of a class waiver, or inclusion of an indication within
a condition
- Discontinuation of paediatric development.
To ensure a smooth transition to using the IRIS platform, it is essential that applicants
prepare well in advance, including registering for IRIS, as described in the following document:
https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/iris-guide-registration-and-rpis_en.pdf
The gateway is open for ongoing paediatric procedures only.
15-05-2024
A new PLM Portal Home page
New, product specific tiles for electronic Product Information (ePI) and the Product
Management Service (PMS) Product User Interface have been included in addition to the web-based
electronic Application Forms on the PLM Portal's landing page. To ensure users can find and easily
access these tools, EMA has updated the landing page of the Portal, which now presents a new look &
feel.
The new interface aims to provide more intuitive access to eAF, PMS and ePI release notes, news,
knowledge articles.
We would like to invite you to share your feedback on the new landing page in the PLM Portal Forum.
AND
Recommended use of web-based human variations eAF for all CAPs
As anticipated in April 2024 communication and announced in May 2024 at the webinar “Information and Q&A session on updated CAPs in web-based eAF” (recording &
presentation available), we have completed the load of updated* Centrally Authorised Products (CAPs)
data to PLM Portal web-based eAF on 19 April 2024. Users can now access this new CAP product data
directly within the web-based forms.
We are pleased to announce that we recommend the use of the PLM Portal web-based eAF for all
CAPs variations starting from 14 May 2024. However, please be aware that
there are still some known issues and limitations that we are actively addressing
however, these issues do not prevent the use of the web-based eAF. We invite you to consult the 7 May
webinar presentation for the complete list of known issues.
If you encounter any issues, please report them via EMA Service Desk,
ensuring that you select the correct category.
*Including split & match-merge processes. Please refer to EU IG (Implementation Guide) Chapter 7 for a detailed description of these
steps.
26-04-2024
Paediatric submissions to launch on IRIS platform from 4 June
2024
Please note that from 4 June 2024, the paediatric submissions must be carried out via IRIS. For more information please see the announcement.
24-04-2024
Register for the Information and Q&A session on updated CAPs in PLM Portal
eAF, 7 May 2024
EMA will host an Information and Q&A session on 7 May 2024, 10:00 - 11:00 (CEST) to explain and showcase the changes in the PLM Portal web-based Human Variations eAF after the updated Centrally Authorised Products (CAPs) load. Participants will have the opportunity to ask questions in the last part of the session.
The participation is recommended to Industry stakeholders working on regulatory affairs of their respective organisations.
Register here, and check the event web page for agenda, presentation and recording, all to be published in due time.
23-04-2024
Successful CAPs data load under review, recommended use of interactive PDF
extended
Updated Centrally Authorised Products (CAPs) data has been loaded to web-based (PLM Portal) eAF, where users will be able to see this data in their forms.
Please note that, while CAPs data is available as of 16 April, we strongly recommend that no applications using the PLM Portal web-based eAF is used for submissions at the moment. This is due to the fact that the updated CAPs load has impacted how products appear in application forms (e.g., changes to product names, introduction of new medicinal products through splitting, potential alterations to packages, etc.) and some minor adjustments to the functionalities of the web based eAF are needed.
We suggest using the interactive PDF eAF instead of the web-based eAF for submissions to prevent validation issues and potential delays. The EMA will announce the date from which the use of the PLM web-based eAF form can be resumed.
19-04-2024
Updated VNeeS checker for v3.1.1 now available
An updated version of the VNeeS checker provided by Anses - ANMV is now available.
11-04-2024
Updated eAF v1.26.0.1 (Vet MAA) now available
An updated v1.26.0.1 Vet MAA eAF is now available on the eSubmission
website.
A change has been implemented to fix a bug related to the navigation in the form after its
finalisation.
It is recommended to use this latest form for new submissions. Please note that there is no
version number change and that the release notes will be updated and published in the relevant section
of the eAF page.
AND
electronic Application Forms (eAFs) not available for use between 11th and 16th April
2024 due to planned maintenance
Due to planned maintenance activities, the interactive PDF eAFs (human and
veterinary variation and MAA forms and the renewal form) and the PLM Portal web-based
eAF will be unavailable for use between 11th and 16th April 2024.
Please refer to the news published on 15 March 2024 for the complete details of the planned
maintenance and we remind you that we strongly recommend to use the interactive pdf eAF
instead of the web-based eAF for submissions at least until 26 April 2024 to prevent
validation issues and potential delays.
20-03-2024
Planned maintenance of the eSubmission systems on 25 March 2024, 18:00 CET
Due to planned maintenance, the following eSubmission systems will not be available on
Monday 25 March 2024, between 18:00 and 21:00: Gateway XML delivery file user
interface and Common Repository Web-UI (relevant for NCAs only).
The maintenance includes the introduction of a new field in the eSubmission Gateway XML
delivery file user interface (EPITT number for pam-sda submissions), and other small bug fixes and
improvements.
Previously created delivery files will not continue to work, please use the new version of the Gateway
XML delivery file after the maintenance has completed.
Updated documentation will be published on the relevant pages of the eSubmission website.
For any further information, please contact EMA Service Desk.
15-03-2024
electronic Application Forms (eAFs) not available for use between 11th and 16th April
2024 due to planned maintenance
Due to planned maintenance activities, the interactive PDF eAFs (human and
veterinary variation and MAA forms and the renewal form) and the PLM Portal web-based
eAF will be unavailable for use between 11th and 16th April 2024.
Additionally, as announced in December 2023, the PLM Portal web-based eAF
will be affected by the data load of Centrally Authorised Products (CAPs) and Nationally Authorised
Products (NAPs) into Product Management Service (PMS) as well as the simultaneous load of updated*
CAPs to web-based eAF.
This load of data into PMS is a necessary step in preparation to the forthcoming launch of the Product
User Interface view and later edit functions as well as making NAPs data available for the PLM Portal
web-based eAF.
The data load will take place from 11 to 16 April 2024.
During this timeframe, the interactive PDF eAF and the PLM Portal web-based eAF will
experience a downtime.
Please note that, during the preparation for the updated* CAPs load in the PLM Portal
web-based eAF, the match-merge** operation will result in:
- changes to product names;
- introduction of new medicinal products through splitting**;
- potential alterations to packages.
These changes will impact how products appear in application forms. Therefore, we
strongly recommend, with immediate effect, that no applications that might
require any update of the eAF, during or after the product upload, are submitted to
EMA using the PLM Portal web-based eAF. We strongly recommend to use the
interactive pdf eAF instead of the web-based eAF for submissions at least until 26 April
2024 to prevent validation issues and potential delays.
Please note that if you have already submitted a web-based eAF, or it is expected that there will be
no need to update the form and/or the procedure will conclude prior to the downtime, you can continue
using the web-based eAF.
Please note that, except for the downtime period, the web-based eAF is expected to remain
accessible to applicants to familiarise themselves with changes to the products, and for training
purposes.
*Including split & match-merge processes. Please refer to EU IG (Implementation Guide) Chapter 7 for a detailed description of these
steps.
**The “Match-merge” process serves to include data from XEVMPD to products already released in PLM
Portal. The “split” process serves to make released products ISO-IDMP compliant. Both processes are
explained in detail in EU IG Chapter 7
15-03-2024
Register for the eCTD v4.0 Vendor Workshop on 27 March 2024
The electronic Common Technical Document (eCTD) is the standard norm for industry
submissions for over 20 years and currently implemented by regulators and industry in version 3.2.2.
Version 4.0 of eCTD was published in December 2015 with its most recent Implementation Package v1.5
endorsed at the May 2022 ICH meeting.
This workshop, organised and facilitated by the eCTD v4.0 SME Group (EMA, NCAs and
Industry), is intended for all eCTD Vendors that are interested in better understanding changes to the
EU regional eCTD Specification. The event aims at fostering a discussion around the key sections of
the new version of the Implementation Guide, the technical understanding of the implementation of the
new standard, including the grouped submissions, document re-use and controlled vocabularies. At the
moment, the focus will be on the Centralised Procedures only.
Moreover, during the workshop, the scope and timeline of the pilot, as well as the timeline
for the updates of the Implementation Guide, will be discussed.
This session also offers an invaluable opportunity for participants to ask questions and
provide feedback on pertinent matters.
Register for this workshop.
After registration you will receive a confirmation e-mail with the link and password to
access the workshop. Please make sure to save those to be able to participate to the workshop.
15-03-2024
Planned maintenance of the Common Repository Torrent in the evening of 21 March
2024
Due to planned maintenance, the use of the Torrent files via client to download the content
will not be possible on Thursday 21 March 2024, between 18:00 and 21:00. For any further information,
please contact EMA Service Desk.
14-03-2024
eCTD v4.0 EU M1 Implementation Guide - draft Version 1.1 now available
A new draft version of the eCTD v4.0 EU M1 Implementation Guide is now
available here for
consultation. Future versions (together with EU Controlled Vocabularies and other annexes) will be
published and announced on the eSubmission website.
29-02-2024
Updated eAF v1.26.0.1 (Vet MAA) now available
An updated v1.26.0.1 Vet MAA eAF is available starting with 29 February 2024, 18:00 CET.
A change has been implemented to add "United Kingdom (Northern Ireland)"" in the Member state selection in sections 2.4.1 and 2.4.4.
It is recommended to use this latest form for new submissions. Please note that there is no version number change and that the release notes will be updated and published in the relevant section of the eAF page.
For the UK, as from 1.1.2021, EU Law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland/NI.
20-02-2024
PLM Portal eAF and PMS - FAQs and Q&A documents updated
The PLM Portal eAF and PMS teams are pleased to announce that the updated Frequently Asked Questions (FAQs) on eAF and PMS and Questions and Answers (Q&A) Joint eAF and PMS are now available here.
02-02-2024
Updated PLM Portal eAF FHIR XML Release notes now available
An updated version of the PLM Portal eAF FHIR XML Release notes reflecting the implementation of FHIR XML 2.0.0 in the PLM Portal eAF (to be released to production on 30 April 2024) are now available on the PLM Portal and in the section of the PLM Portal eAF, Documentation section, together with several documents to provide consumers of the FHIR XML message the easiest possible way to upgrade to the eAF XML 2.0.0 or to start the implementation now with the latest version.
The new version includes the latest official FHIR version “Release #5 (5.0.0): http://hl7.org/fhir/”. This replaces the current version FHIR Release #5: Draft Ballot (4.6.0): http://hl7.org/fhir/2021May/ which will remain online for approximately 3 months to give consuming systems time to adapt to the change.
02-02-2024
User Experience of the current PDF eAFs completion on the PLM Portal
The electronic Application Form (eAF) Product Team would greatly appreciate to receive your feedback on your current experience in terms of time spent in filling in the interactive pdf electronic Application Form (eAF) for Variations for Centrally Authorised Products (CAPs) and Nationally Authorised Products (NAPs), and Initial Marketing Authorisation.
It is acknowledged that different types of procedures (e.g. Type IA, Type II applications, worksharing, and groupings) may require varying time commitments. We would therefore appreciate if, in your answer, you could provide the average time spent and resources used to fill in the forms.
Your feedback will provide us an indication on the current performance requirements and will be our starting point for improving future user experience.
You can find the questionnaire to fill in at the following link:
https://ec.europa.eu/eusurvey/runner/Eusurvey_PLMPortal_UX_eAFcompletion
Please note that this survey should not take more than 5 minutes to complete, and the responses will remain anonymous.
We kindly ask you to respond to the survey by Thursday 29 February 2024.
Please send any question to plm.valuestream@ema.europa.eu or post them via the PLM Portal Forum.
18-01-2024
A new version of the eSubmission Gateway XML delivery file for veterinary Variations Requiring Assessment (VRA) now available
An updated version of the eSubmission Gateway XML delivery file user interface for veterinary VRA submissions is available starting with 17 January 2024, 18:00 CET.
This update introduced a split of the submission type VRA to 4 different submission types according to the Timetable as per defined in the Variation Classification document.
The users should note that they should always select the standard timetable (E/S/R) for each scope, regardless of a different TT having been agreed.
For grouped VRA, in the delivery file, the applicant should select the longest timetable applicable to any of the included variations in the application.
Updated release notes and user guidance are published in the relevant section.
Users should note that the delivery files created prior to the new release will not work after the go live.
15-01-2024
A new version of the eSubmission Gateway XML delivery file for veterinary Variations Requiring Assessment (VRA) is planned for release in the evening of 17 January 2024
An updated version of the eSubmission Gateway XML delivery file user interface for veterinary VRA submissions is planned for release in the evening of 17th January 2024. This update will introduce a split of the submission type VRA to 4 different submission types according to the Timetable as per defined in the Variation Classification document
The users should note that they should always select the standard timetable (E/S/R) for each scope, regardless of a different TT having been agreed.
For grouped VRA, in the delivery file, the applicant should select the longest timetable applicable to any of the included variations in the application.
Users should note that the delivery files created prior to the new release will not work after the go live.
And
A new version of the eSubmission Gateway XML delivery file for SEND packages now available
An updated version of the eSubmission Gateway XML delivery file user interface is available starting with 11 January 2024, 18:00 CET. This update introduced in the delivery file for Human submissions the option to specify if there is a “SEND Data package Included”.
Updated release notes and user guidance are published in the relevant section.
Users should note that the delivery files created prior to the new release will not work after the go live.
10-01-2024
Opportunity to submit SEND data packages with new Market Authorisation Applications
From January 2024, EMA is launching a proof-of-concept study to evaluate the added value of using SEND data in the evaluation of new Marketing Authorisation Applications. Applicants are encouraged to submit their SEND data packages, in addition to the eCDT format, as part of their MAA submission. The SEND package must be provided outside the eCTD, inside the working documents folder to avoid eCTD technical validation failure.
An updated version of the eSubmission Gateway XML delivery file user interface will be available starting with 11 January 2024, 18:00 CET. This update introduces in the delivery file for Human submissions the option to specify if there is a “SEND Data package Included”.
Users should note that the delivery files created prior to the new release will not work after the go live.
What is SEND?
SEND is the Standard for Exchange of Nonclinical Data between organisations, which provides a standardised format for the submission of nonclinical data to regulatory bodies.
SEND was created by the Clinical Data Interchange Standards Consortium (CDISC) in 2002 to execute on the Study Data Tabulation Model (SDTM) for the submission of nonclinical studies. A SEND dataset package contains the SEND datasets (.xpt files), the Nonclinical Study Data Reviewer's Guide (nsdrg.pdf), and the Define XML Document (define.xml).
Why a proof-of concept?
Standardisation of data presentation has been shown to significantly reduce the time regulators require for reviewing the non-clinical data packages. In this proof-of-concept study, we will evaluate whether using SEND data in the assessment of the non-clinical dossier will lead to improved and more consistent quality of assessments, to more science-driven questions to Applicants, and to faster completion of the non-clinical dossier assessment.
Any questions? Email us at send@ema.europa.eu
21-12-2023
eCTD v4.0 Vendor Workshop Survey
EMA, together with the eCTD v4.0 EU Subject Matter Experts group, is planning a Vendor Workshop in Q1 2024. To assess the availability, interest and further topics for discussion, the Vendors are invited to complete the eCTD v4.0 Vendor Workshop survey by end of day 15 February 2024.
20-12-2023
Update on web-based Human variations electronic Application Forms (eAFs) implementation
As anticipated in October 2023, the electronic Application Form (eAF) and Product Management Service (PMS) teams would like to provide you with an update on the progress of the web-based Human variations eAF implementation on the Product Lifecycle Management (PLM) Portal
During Q4 2023, we completed the load and evaluation of PMS data in PMS User Acceptance Testing (UAT) environment, after which we proceeded to testing of PMS data in the PLM Portal. While performing these activities, we observed the following:
- Bugs in PMS related to match-merge* of Centrally Authorised Products (CAPs);
- Technical issues with the eAF preventing testing;
- System performance improvements required for the PLM Portal.
Taking into consideration these findings, we have made the necessary adjustments to planning. Kindly find here a visual representation of the updated plan.
Please note that Alpha UAT for Product User Interface (UI) data quality is still ongoing and proved successful thus far, as no blocking bugs have been identified.
Key points of the updated plan:
Q1 2024:
- Deployment in production of new eAF features (e.g., clone application, add package, rename application) developed in Q4 2023.
- Consolidation of all PLM portal products' development (eAF, Product UI, ePI) under a single service provider.
Q2 2024:
- Release of all CAPs & NAPs in the PMS database. Simultaneously, release of updated** CAPs in eAF;
- Delivery of PMS Application Programming Interface (API) - with CAPs and Nationally Authorised Products (NAPs) available in view-only mode - and Product UI - with CAPs available in view-only mode and NAPs not yet viewable.
Please note that, during the preparation for the updated** CAPs load in eAF in Q2 2024, the match-merge* operation will result in:
- changes to product names;
- introduction of new medicinal products through splitting*;
- potential alterations to packages;
This will impact how products appear in application forms. Therefore, we will communicate in due course the 3-week period where it is advisable to use the interactive eAF (PDF) instead of the web-based eAF for submissions to prevent validation issues and potential delays. Please note the web-based eAF will remain accessible to applicants to familiarise themselves with it and for training purposes.
Q3 2024:
- Performance improvements and internal testing
Q4 2024:
- Release of NAPs in the eAF
The updated planning does not foresee in 2024:
- external testing of the eAF UAT
- consequently, no transition start towards mandatory use of the Human variations web-based eAF. However, we would recommend the use of the web-based eAF for CAPs submissions following the updated** CAPs release.
Please send any questions to eSubProgofficer@ema.europa.eu or post them via the PLM Portal Forum.
*The “Match-merge” process serves to include data from XEVMPD to products already released in PLM Portal. The “split” process serves to make released products ISO-IDMP compliant. Both processes are explained in detail in EU IG Chapter 7
**Including split & match-merge processes. Please refer to EU IG Chapter 7 for a detailed description of these steps.
07-12-2023
Updated eAF v1.26.0.0 (Human MAA) and v1.26.0.1 (Vet MAA) now available
Updated v1.26.0.0 Human MAA eAF is now available on the eAF website. The form is ready for immediate use. The change implemented in this version is a minor bug fix (related to Annex 5.19).
It is strongly recommended to use this latest version (document properties date 27.10.2023). Please note that there is no version number change and that the release notes are published in the relevant section of the eAF webpage.
Updated v1.26.0.1 Vet MAA eAF is now available for immediate use.
The changes in this version relate to label and text changes only. Additionally, the version number has updated from v1.26.0.0 to v1.26.0.1.
The version 1.26.0.1 can be used immediately, and it is strongly recommended that it will be used for all new applications as soon as possible, however, the one-month transitional period will run until 8th of January 2024 after which the use of version 1.26.0.1 for the Veterinary MAA form will become mandatory.
The version 1.26.0.0 has now been removed from the eAF website however users can continue to submit applications using this version until 7th of January 2024.
Please note that as there are no technical changes, it is possible to import the xml from version 1.26.0.0 into version 1.26.0.1 to avoid any need for manual effort.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure, please note that if you need to provide an updated form for a procedure that has started prior to 8th of January, you should use the previous version.
Updated release notes are published in the relevant sections of the eAF webpage.
05-12-2023
Survey for eSubmission website (open until Monday, 18 December 2023)
In light of an ongoing application study at the European Medicines Agency, we are gathering feedback on the eSubmission website.
By completing this short 2-3 minutes survey, you help us to understand if the website addresses your user needs.
May we kindly ask you to complete this survey by Monday, December 18th? The link to the survey can be found here: eSubmission User Survey.
We highly appreciate your input.
And
05-12-2023
Survey for PSUR Repository (open until Monday, 18 December 2023)
In light of an ongoing application study at the European Medicines Agency, we are gathering feedback on the PSUR Repository.
By completing this short 2-3 minutes survey, you help us understand if the application addresses your user needs.
May we kindly ask you to complete this survey by Monday, December 18th? The link to the survey can be found here: PSUR Repository User Survey.
We highly appreciate your input.
01-12-2023
New submission unit to be used for requesting a Re-examination of an CHMP Opinion
An updated version of the eSubmission Gateway XML delivery file user interface will be available starting with 1 December 2023, 18:00 CET. This update introduces in the delivery file for Human submissions the option to choose "Re-examination" submission unit for various submission types. Use this unit for requesting re-examination of opinion for MAA, extension, Type II variation, renewal and annual re-assessment as well as Referral procedures
Please note that regulatory guidance referring to how to send re-examination requests will be updated in the following period. Re-examination requests should be submitted via the eSubmission (Syncplicity) Gateway using eCTD format where required for the procedure type.
30-11-2023
Updated version of the eAF v1.26.0.0 (variation and renewal)
An updated version 1.26.0.0 of the Variation and Renewal (both Human and Veterinary) eAF is available starting with 28 November 2023, 18:00 CET.
A change has been implemented to allow Non-Current terms to be selected in the "Pharmaceutiacal form" lists.
It is recommended to use this latest form for new submissions. Please note that there is no version number change and that the release notes were updated and published in the relevant section of the eAF page.
28-11-2023
eCTD EU Module 1 Specification proposed changes now open for public consultation (Deadline: 12 January 2024)
The Human Harmonisation Group has analysed the change requests received upon opening the eCTD EU Module 1 Specification for review and is now publishing the draft documents for public consultation:
The eCTD EU Module 1 Specification contains a summary of the proposed changes in the Document control table, and all the amendments are highlighted throughout the text with the track changes feature.
The eCTD EU Validation Criteria contains a summary of the proposed changes in the Document control tab.
Please submit your questions and comments via email to EUM1Spec@ema.europa.eu by the end of the day 12 January 2024.
After consolidating the public comments, the final versions of the documents and all the relevant files (including the Release notes, Package, Checksums) will be published on the eSubmission website. The date of entering into force will be announced at the time of the publication and will allow 9 months for the implementation of the changes.
28-11-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.12 released to production on 27 November 2023 are now available on PLM Portal Forum and on the PLM Portal eAF web page.
20-11-2023
Invitation to register for the Product Lifecycle Management Value Stream Deep-Dive Webinar, 30/11/23 h. 14:00-16:00 (CET)
EMA is pleased to announce a public webinar (event page on EMA website) for medicines developers and regulators eager to gain deeper insights into what the Agency's Product Lifecycle Management (PLM) value stream is working on and its goals. The event will take place on 30 November, 14:00 - 16:00 (CET).
This webinar aims to show the interconnections between the various digital products being delivered. In addition, it will outline the approach taken to enhance product lifecycle management technology to enable more efficient and effective medicine regulation.
About PLM:
PLM is one of three value streams covering the product lifecycle implemented by the Agency as part of its Agile transformation. PLM aims to digitally transform and optimise regulatory procedure management as well as data submission and reuse throughout the product lifecycle. The value stream works with EMA partners and stakeholders to deliver systems and services to manage the authorisation and lifecycle of medicinal products and medical devices within the Agency's remit for the ultimate benefit of public and animal health in the EU.
About the Webinar:
During the webinar, the team will share the value stream's vision and roadmap towards a data centric target operating model for end-to-end data capture, processing and re-use, as well as how PLM's products and solutions contribute to this vision.
Featured PLM Products and Solutions:
- Solutions for Human & Veterinary medicinal products:
- Solutions for Human medicinal products:
- Solutions for Veterinary medicinal products:
What's in it for you:
This webinar presents a valuable opportunity to enhance your understanding of the work of the value stream and stay informed about the latest developments in this area, ask questions and interact with the responsible EMA teams.
Registration & Contact details:
Interested stakeholders are invited to kindly register at the following link.
Please note registration will remain open until the start of the webinar.
You have the opportunity to send your questions in advance via Slido.com using the specific event code: #PLMVS-QA.
Should you have any questions or require further information, please feel free to contact us at plm.valuestream@ema.europa.eu.
14-11-2023
Updated PLM Portal eAF FHIR XML Release notes now available
An updated version of the PLM Portal eAF FHIR XML Release notes reflecting the implementation of FHIR XML 1.2.0 in the PLM Portal eAF (to be released to production on 21 November 2023) are now available on PLM Portal Forum
23-10-2023
Updated VNeeS checker for v3.1 now available
An updated version of the VNeeS checker provided by Anses - ANMV is now available here
19-10-2023
Updated variation Conceptual Data Model
The conceptual data model for the variation form has been updated and is now available here
06-10-2023
Updated version of the VNeeS specification v3.1 now available
The guideline on eSubmissions for Veterinary products version 3.1 has now been published. It will enter into force on 1 November 2023 (see links under section “Current Guidance”).
The update is related to a best practice criterion in the technical validation checklist for the additional information folder (“add-info”). In case you will be using a checker tool not yet aligned with this new best practice requirement, for relevant submissions like SRPs, in case of doubt, we recommend to verify the path length in the add-info folder with the publicly available VNeeS checker tool to avoid potential technical blocks during dossier upload and thus circumvent the need for last minute changes of your submission.
06-10-2023
eSubmission Gateway XML delivery file update for Article 18 submissions
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced a small change to the delivery file for Human submissions (option to choose Article 18 as submission type).
The details on the changes will soon be provided in the updated release notes and the updated user guide. More information on Article 18 can be found
here.
05-10-2023
Update on web-based electronic Application Forms (eAFs) implementation
The electronic Application Form (eAF) and Product Management Service (PMS) teams would like to provide you with a comprehensive update on the progress of the web-based Human variations eAF implementation on Product Lifecycle Management (PLM) Portal.
The following milestones are now for Q1 2024:
- Release of split* (i.e. IDMP compliant) Centrally Authorised Products (CAPs) for use in the variations eAF;
Release National Authorised Products (NAPs) for use into the Human variations eAF;
Introduction of the Product Data Management User Interface (UI), offering users seamless access to view their product data available in the Product Management Services (PMS) database.
As we advance, we remain committed to transparency and will provide a comprehensive update on these targets at the end of Q4 2023, evaluating the quality of both the system and available data.
Currently, our primary focus is ensuring quality of data and system's performance to prepare a transition-ready version of the web-based Human variations eAF. To begin the transition phase towards mandatory use of web-based Human variations eAFs, the following key steps are required:
- Implementation of missing features.
- Resolving pending issues that prevent users from completing a form.
- Releasing all products (NAPs and split* (i.e. IDMP compliant) CAPs) from the Product Management System (PMS) to the PLM portal in the production environment.
- Capability to keep PMS data synchronised with existing databases.
- Ensuring system stability.
As previously committed, any announcement concerning the starting date of the formal transition period will follow User Acceptance Testing (UAT) of the system and the subsequent addressing of any critical issues. This is explained in this slide.
The primary objective remains to implement web replacements of interactive PDF eAFs, enabling user-friendly capture and handling of variations, marketing authorisations, and renewals application data for both applicants and regulators. This transition ensures consistency across IT systems and guarantees the availability of high-quality ISO IDMP compliant information.
For further details on the implementation progress and challenges, and the opportunity to ask questions, interested parties are invited to the joint eAF-PMS webinar for 6 November 2023 (13:30 - 15:00 (CEST)). During this session, the eAF team will also demonstrate the anticipated "add package" and "clone application" features that will be released on the PLM Portal for eAF. Please find here the registration link.
We also recommend that anyone with an interest in the development of eAF and PMS watch the relevant sections of the Q3 System Demo recording, available on EMA's website.
Please send any questions to eSubProgofficer@ema.europa.eu or via the PLM Portal Forum.
*CAPs migrated from SIAMED not following ISO IDMP structure. For this reason, they have undergone a further step in the data migration to PMS in addition to the match and merge protocol.
04-10-2023
Updated user guide for the eAF for MAA available on the CMDh website
A new version of the joint EMA/CMDh User guide for the electronic application form for a Marketing Authorisation is available on the CMDh website.
13-09-2023
Planned maintenance of the PSUR Repository (NCA and Industry) on 16 September 2023
Due to planned maintenance, the PSUR Repository (both NCA and Industry portals) will not be available on Saturday, 16 September 2023, between 9:00 and 17:00. For any further information, please contact EMA Service Desk
29-08-2023
Planned maintenance of the PSUR Repository (NCA and Industry) on 2 September 2023
Due to planned maintenance, the PSUR Repository (both NCA and Industry portals) will not be available on Saturday,2 September 2023, between 9:00 and 17:00. For any further information, please contact EMA Service Desk
10-08-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.11 released to production on the 07 of August 2023 are now available on PLM Portal Forum
01-08-2023
Updated PLM Portal eAF Guide to Registration is now available
Please find an updated version of the PLM Portal eAF Guide to Registration here.
27-07-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.10 released to production on the 26 of July 2023 are now available on PLM Portal Forum and on the PLM Portal eAF web page.
12-07-2023
Human variations web-based eAF Timeline Review
We inform you that the timeline of the variations web-based electronic Application Form (eAF) for Human medicinal products has been updated.
EMA is continuing development work with the aim to incrementally build the new web-based eAF, leveraging on Product Management Service (PMS) data. The key priority for the Agency is to ensure that the eAF is supported by a stable system with high-quality data before starting any transition. During recent testing, it became clear that the target date for the release of split CAPs and NAPs on the PLM Portal requires to be extended. This is due to the additional time needed to perform checks on data quality and the quality of the data transfer of NAPs data, investigate and address potential issues connected to product data transfer and (if needed) repeat the loading of the data.
Therefore, in order to respect our commitment to ensuring that all stakeholders are kept informed on key milestones with reasonable advance notice, please be informed of the new target dates for the release of split Centrally Authorised Products (CAPs) and Nationally Authorised Products (NAPs) for use in web-based eAFs on the Product Lifecycle Management (PLM) Portal:
- release of split CAPs, making them available to use in the eAF, is now expected in October 2023 instead of July-August 2023
target date for the release of NAPs is now expected between November 2023 and February 2024 instead of July-August 2023.
Consequently, the human variations web-based electronic Application Form (eAF) target timeline (last published in April 2023) has been reviewed. Please find here the updated version, which reflects the target dates for each milestone. Kindly note the eAF team expects to provide a further update to confirm the timeline in September 2023, based on latest testing outcomes.
Please be informed that key milestones following split CAPs and NAPs release in web-based eAF on the PLM Portal will be consequently rescheduled. In particular, the external User Acceptance Testing on the version of the web-based eAF intended to replace the PDF and trigger the transition will be rescheduled from November 2023 to a yet-to-be-defined date which will be announced at least two months in advance, as per prior commitment. The transition period will also be announced in due time.
Please note that EMA is following the previously communicated development steps for the products' release on the PLM Portal:
- Load product data into the test environment;
- Test data quality and quality of the transfer;
- Investigate issues, address them and repeat the first 2 steps;
- Once ready, transfer product data into the production environment.
In the meantime, applicants are encouraged to use the human variations web-based eAF already available in the PLM Portal for products already in the system and maintain high the level of data quality of their products in XEVMPD.
Please send any questions to eSubProgofficer@ema.europa.eu or via the PLM Portal Forum.
12-07-2023
Human variations web-based eAF Timeline Review
We inform you that the timeline of the variations web-based electronic Application Form (eAF) for Human medicinal products has been updated.
EMA is continuing development work with the aim to incrementally build the new web-based eAF, leveraging on Product Management Service (PMS) data. The key priority for the Agency is to ensure that the eAF is supported by a stable system with high-quality data before starting any transition. During recent testing, it became clear that the target date for the release of split CAPs and NAPs on the PLM Portal requires to be extended. This is due to the additional time needed to perform checks on data quality and the quality of the data transfer of NAPs data, investigate and address potential issues connected to product data transfer and (if needed) repeat the loading of the data.
Therefore, in order to respect our commitment to ensuring that all stakeholders are kept informed on key milestones with reasonable advance notice, please be informed of the new target dates for the release of split Centrally Authorised Products (CAPs) and Nationally Authorised Products (NAPs) for use in web-based eAFs on the Product Lifecycle Management (PLM) Portal:
- release of split CAPs, making them available to use in the eAF, is now expected in October 2023 instead of July-August 2023
target date for the release of NAPs is now expected between November 2023 and February 2024 instead of July-August 2023.
Consequently, the human variations web-based electronic Application Form (eAF) target timeline (last published in April 2023) has been reviewed. Please find here the updated version, which reflects the target dates for each milestone. Kindly note the eAF team expects to provide a further update to confirm the timeline in September 2023, based on latest testing outcomes.
Please be informed that key milestones following split CAPs and NAPs release in web-based eAF on the PLM Portal will be consequently rescheduled. In particular, the external User Acceptance Testing on the version of the web-based eAF intended to replace the PDF and trigger the transition will be rescheduled from November 2023 to a yet-to-be-defined date which will be announced at least two months in advance, as per prior commitment. The transition period will also be announced in due time.
Please note that EMA is following the previously communicated development steps for the products' release on the PLM Portal:
- Load product data into the test environment;
- Test data quality and quality of the transfer;
- Investigate issues, address them and repeat the first 2 steps;
- Once ready, transfer product data into the production environment.
In the meantime, applicants are encouraged to use the human variations web-based eAF already available in the PLM Portal for products already in the system and maintain high the level of data quality of their products in XEVMPD.
Please send any questions to eSubProgofficer@ema.europa.eu or via the PLM Portal Forum.
11-07-2023
Common repository Go-live (relevant for NCAs only)
We are pleased to inform you that the next version of the Common Repository introducing the 'Basic Authentication' is going live on the >18th July 2023.
This release improves security by modifying access to the Common Repository requiring all users to log in to the system to view/download submissions. Additionally, this release provides usability improvements mainly related to procedures containing Nationally Authorised Products.
For the completion of the deployment, Common Repository UI and API and eSubmission Delivery File UI will be unavailable on Tuesday evening, 18th of July 2023 between 18:00 and 19:30 (CET).
11-07-2023
Updated draft eCTD v4.0 implementation timeline for EU now available
An updated draft timeline for the implementation of eCTD v4.0 in the EU is now available on the
eCTD v4.0 page here.
04-07-2023
A new version of the PSUR Repository NCA UI is now available
An updated version of the PSUR Repository NCA UI is now available. The new version of the PSUR Repository NCA User Interface provides a technical update only. Following the update, the PSUR Repository NCA UI has slightly different look and feel, however, there are no changes to the features and functionality of the system.
User Guidance and the Release Notes were updated and they can be found in the “User Documents” section of the page.
30-06-2023
Planned maintenance of the Common Repository on 5 July 2023
Due to planned maintenance and verification of the upcoming functionalities (including basic authentication), the Common Repository (API and Web UI) will not be available on Tuesday 5 July 2023, between 18:00 and 21:00. For any further information, please contact EMA Service Desk.
28-06-2023
PSUR Repository (NCA UI) Go-live 3rd July 2023
We are pleased to inform you that an updated version of the PSUR Repository NCA UI is going live on the 3rd of July 2023.
The new version of the PSUR Repository NCA User Interface provides a technical update only. Following the update, the PSUR Repository NCA UI will have slightly different look and feel, however, there are no changes to the features and functionality of the system.
For the completion of the deployment, the PSUR Repository NCA User Interface will be unavailable on Monday evening 3rd of July 2023 between 18:30 and 19:30 (CET).
And
Updated regulatory practical guidance
The European Medicines Agency practical guidance on the application form for centralised type IA and IB variations (europa.eu) has been updated and can also be found under the section “Regulatory”.
27-06-2023
Planned maintenance of the Common Repository on 27 June 2023
Due to planned maintenance and verification of the upcoming functionalities (including basic authentication), the Common Repository (API and Web UI) will not be available on Tuesday 27 June 2023, between 18:00 and 21:00. For any further information, please contact EMA Service Desk.
22-06-2023
Planned maintenance of the eSubmission systems on 24 June 2023
Due to planned maintenance, the following eSubmission systems will not be available on Saturday 24 June 2023, between 8:00 and 18:00: Common Repository (Interface and API), PSUR Repository, Gateway FileHandler. For any further information, please contact EMA Service Desk.
21-06-2023
Planned maintenance of the PSUR Repository on 22 June 2023
Due to planned maintenance, the PSUR Repository will be available intermittently on Thursday 22 June 2023, between 17:00 and 20:00. For any further information, please contact EMA Service Desk.
20-06-2023
eSubmission Gateway XML delivery file update for Real World Data
An updated version of the eSubmission Gateway XML delivery file user interface is available starting with 20 June 2023, 18:00. This update introduces a change to the Human Domain only, adding new fields to flag Real World Data/Real World Evidence for selected types of submissions.
The details on the changes are provided in the related release notes which are available here and the updated user guide is available here.
Previously created delivery files should continue to work; however, it is always recommended to clear the cookies and the cache to ensure that the system works correctly.
And
Planned maintenance of the Common Repository on 20 June 2023
Due to planned maintenance, the Common Repository (User interface and API) will not be available on Tuesday 20 June 2023, between 18:00 and 20:00. For any further information, please contact EMA Service Desk.
19-06-2023
Planned maintenance of the PSUR Repository on 19 June 2023
Due to planned maintenance, the PSUR Repository will be available intermittently on Monday 19 June 2023, between 18:00 and 22:00. For any further information, please contact EMA Service Desk.
And
Planned maintenance of the Common Repository on 20 June 2023
Due to planned maintenance, the Common Repository (User interface and API) will not be available on Tuesday 20 June 2023, between 18:00 and 20:00. For any further information, please contact EMA Service Desk.
15-06-2023
Planned maintenance of the eSubmission systems on 17 June 2023
Due to planned maintenance, the eSubmission systems (including eAF and PLM Portal eAF) will not be available on Saturday 17 June 2023, between 8:00 and 16:00. For any further information, please contact EMA Service Desk.
And
Updated version of the eAF v1.26.0.0 (human variation)
An updated version 1.26.0.0 of the human variation eAFs is available starting with 14 June 2023.
A single change has been implemented to address a non-blocking regression bug (related to the declaration on the manufacturers and MAH).
It is recommended to use this latest form for new submissions (document properties date 14.06.2023). Please note that there is no version number change and that the release notes are not published for this minor change at this time.
13-06-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.9 released to production on the 12 of June 2023 are now available on PLM Portal Forum and on the PLM Portal eAF web page.
06-06-2023
Planned maintenance of the eSubmission systems on 9 June 2023
Due to planned maintenance of the eSubmission systems on Friday 9 June 2023, between 18:00 and 21:00 no submissions will be processed, therefore avoid sending submissions during this maintenance window. For any further information, please contact Service Desk
01-06-2023
eSubmission Gateway XML delivery file update for obsolete Veterinary submission types
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced a small change to the delivery file for Veterinary submissions (removal of obsolete submission types: extension, var-type1b, var-type2 and vet-psur).
The details on the changes are provided in the related release notes which are available here and the updated user guide is available here.
30-05-2023
Planned maintenance of the eSubmission systems on 31 May 2023
Due to planned maintenance of the eSubmission systems on Wednesday 31 May 2023, the Delivery File UI and the Gateway FileHandler will be unavailable between 18:00 and 20:00. During this time, submissions uploaded through Syncplicity will not be processed, therefore avoid sending submissions during this maintenance window. For any further information, please contact Service Desk
And
Updated version of the eAF v1.26.0.0 (human variation)
An updated version 1.26.0.0 of the human variation eAFs will be available starting with 31 May 2023, 20:00.
A single change has been implemented to address a non-blocking bug. There is a very limited impact to users of the forms. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human).
It is recommended to use this latest form for new submissions (document properties date 25.05.2023), however you can finalise the existing forms (if any) in the previous version. Please note that there is no version number change and that the release notes are not published for this minor change at this time.
17-05-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.8 released to production on the 16th of May 2023 are now available on PLM Portal Forum and on the PLM Portal eAF web page.
16-05-2023
eAF-PMS Newsletter Issue 3
We are pleased to announce the third edition of the eAF-PMS newsletter is now available at the following link.
This newsletter, available to pharmaceutical companies and National Competent Authorities (NCAs), offers updates on the progress of eAF and PMS, presenting an overview of past and future activities.
In particular, we would like to highlight the following topics:
Upcoming webinars (more details on single events are available in the newsletter):
- Product Lifecycle Management (PLM) Portal Access Management Training Session (25 May 2023, 11:00 - 12:30 Central European Summer Time (CEST)): registration link
- Product Management Service (PMS) Progress Webinar (30 May 2023, 10:00 - 11:30 CEST): registration link
- eAF-PMS Joint Webinar on Nationally Authorised Products (NAPs) Data Release on PLM Portal eAF (8 June 2023, 10:00 - 11:30 CEST): registration link
Updated documents:
Human Variations eAF-PMS Frequently Asked Questions (FAQs) document
Please send any questions to eSubProgofficer@ema.europa.eu or via the PLM Portal Forum.
10-05-2023
eAF PDF working as expected
The selection of active substances is now possible and eAF PDF is working as expected. Should you encounter any issues, please raise a ticket in EMA Service Desk (https://support.ema.europa.eu/esc).
08-05-2023
eAF PDF not working as expected
Please note the eAF PDF is not working as expected (the active substances cannot be selected from the database). Our colleagues are working on fixing the issue and once the eAF PDF will be fully functional, a message will be posted on this page and the users who have raised a related ticket in EMA Service Desk will be contacted as soon as possible.
24-04-2023
Web-based Human Variations eAF (DADI) - Updated timeline
We are pleased to inform you the updated PLM (Product Lifecycle Management) Portal web-based electronic Application Form (eAF) for Human medicinal products release timeline is available on the PLM portal Forum at the following link. In addition, we invite PLM Portal users' feedback on the Portal and web-based eAFs through a survey you can find below.
Note in particular the following periods:
| June 2023 |
Start of data transfer - Centrally Authorised Products (CAPs) and Nationally Authorised Products (NAPs) for use in eAFs - the first NAPs products will appear in the eAF. This will allow users to start preparing web-based eAFs for NAPs as these products become gradually available on the PLM Portal; |
| August 2023 |
Expected completion of data transfer - all CAPs and NAPs available in eAF; |
| November 2023 |
User Acceptance Testing (UAT) on the version of the web-based eAF intended to replace the PDF and trigger the transition; |
| Q1 2024 |
Confirmation of transition period start date, approximately 2 months in |
| Q2 2024 |
Start of 6-months transition to mandatory use of web-based eAF for Human Variations. This is subject to a successful outcome of the UAT. Note that the transition period may start earlier, if feasible, respecting the 2- month advance notice period. |
(Further detailed information on impacts for eAF Users, can be found in the 2nd page of the document link.
Please note:
- Functionalities required for mandatory use are being released incrementally: all required functionalities will be available on the PLM Portal before the start of the UAT.
The use of the web-based eAF will follow the current process for updating data. Use of structured data will be implemented at a later point.
The capability to view migrated PMS product data in the Product User Interface on the PLM Portal is under development in parallel - delivery timelines will be announced at a later date.
Details on how the UAT will be organized will be shared in due course.
and
24-04-2023
Feedback Survey for PLM Portal Users (New Deadline: 8 May 2023)
We kindly ask you to provide your feedback on your experience with the new eAF and the PLM Portal, by responding the survey at the following link: https://ec.europa.eu/eusurvey/runner/Adoption-survey-eAF-HumanVariations
This survey aims to gather your feedback on the level of adoption of the change, including the potential concerns or issues you are facing, in order to enable the eAF product team to envisage appropriate fixing and/or mitigation activities.
Please note that the survey will be anonymous, and the results will not be publicly disclosed.
This survey was already shared with PLM portal users in March 2023 and was intended to close on 31 March 2023, but the deadline for responding is now extended to 8 May 2023.
For more insight on eAF, you are invited to consult the following Q&A documents and sessions:
Training Q&A document (link) addressing questions raised by users during earlier eAF (DADI) trainings;
Human Variations eAF go-live Q&A session (link);
Human Variations eAF go-live training session (2 February 2023) (link).
eSubProgofficer@ema.europa.eu or via the PLM Portal Forum.
19-04-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.7 released to production on the 18th of April 2023 are now available on PLM Portal Forum and on the PLM Portal eAF (DADI) web page.
11-04-2023
PSUR unavailable this Tuesday evening
Due to essential maintenance, PSUR will be unavailable on Tuesday 11 April 2023 between 18:00 and 20:30 (CET). For any questions or concerns, please log a ticket via the EMA Service Desk
21-03-2023
Feedback Survey for PLM Portal Users (Deadline: 31 March 2023)
Following the launch of the Human Variation electronic Application Form (eAF) for Centrally Authorised Products (CAPs) on the Product Lifecycle Management (PLM) Portal, we appreciated many users have accessed the PLM Portal to work on variation applications using the new web-form.
We kindly ask you to provide your feedback on your experience with the new eAF and the PLM Portal, by responding the survey at the following link: https://ec.europa.eu/eusurvey/runner/Adoption-survey-eAF-HumanVariations
This survey aims to gather your feedback on the level of adoption of the change, including the potential concerns or issues you are facing, in order to enable the eAF product team to envisage appropriate fixing and/or mitigation activities.
Please note that the survey will be anonymous and the results will not be publicly disclosed. The survey will stay open until 31 March 2023.
This should not take more than 5 minutes.
17-03-2023
Common Repository Go-Live on 20th of March 2023 (relevant for NCAs only)
We are pleased to inform you that the next version of the Common Repository introducing the 'Basic Authentication' is going live on the 20th of March 2023 (originally planned for the 14th of March). This release improves security by modifying access to the Common Repository requiring all users to log in to the system to view/download submissions. Additionally, this release provides usability improvements mainly related to procedures containing Nationally Authorised Products.
And
Common Repository UI and API and eSubmission Delivery File UI will be unavailable on Monday evening
Common Repository UI and API and eSubmission Delivery File UI will be unavailable on Monday evening.
Due to planned maintenance, Common Repository UI and API and eSubmission Delivery File UI will be unavailable on Monday evening, 20th of March 2023 between 17:30 and 20:30 (CET). For any further information, please contact Service Desk
And
Planned maintenance of the eSubmission systems on Saturday 25 March 2023
The eSubmission systems are unavailable for planned maintenance and will be unavailable from Saturday 25th of March 2023 08:00 until 18:00 due to essential maintenance. For any further information, please contact Service Desk
14-03-2023
Common Repository Go-Live (relevant for NCAs only)
We are pleased to inform you that the next version of the Common Repository introducing the ‘Basic Authentication’ is going live on the 14th of March 2023. This release improves security by modifying access to the Common Repository requiring all users to log in to the system to view/download submissions. Additionally, this release provides usability improvements mainly related to procedures containing Nationally Authorised Products.
For the completion of the deployment, Common Repository UI and API and eSubmission Delivery File UI will be unavailable this Tuesday evening, 14th of March 2023 between 18:30 and 19:30 (CET).
09-03-2023
Updated variation FHIR mapping and conceptual data models
The Variation FHIR mapping and the conceptual data models have been updated and are now available here.
08-03-2023
eAF-PMS FAQs Document Publication
The eAF and PMS teams are pleased to announce that an eAF-PMS FAQs Document is now available here. This document contains Frequently Asked Questions on eAF and PMS and will be updated regularly.
28-02-2023
Common Repository and eSubmission Logs unavailable this Tuesday evening
Due to essential maintenance, Common Repository and eSubmission Logs, will be unavailable on Tuesday 28 February 2023 between 18:00 and 20:00 (CET). For any questions or concerns, please log a ticket via the EMA Service Desk
And
PSUR unavailable this Wednesday evening
Due to essential maintenance, PSUR , will be unavailable on Wednesday 01 March 2023 between 18:00 and 20:00 (CET). For any questions or concerns, please log a ticket via the EMA Service Desk
22-2-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.6 released to production on 21st of February 2023 are now available on PLM Portal Forum and on the PLM Portal eAF (DADI) web page.
24-1-2022
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.4 released to production on 23rd January 2023 are now available on the PLM Portal Forum and on the PLM Portal eAF (DADI) web page.
And
Updated PLM Portal Guide to Navigation is now available
Please find an updated version of the PLM Portal eAF Guide to Navigation here. The slide is now available on the PLM Portal Forum and on the PLM Portal eAF (DADI) web page.
And
eAF - PMS Newsletter Issue 2
We are pleased to inform you that the second edition of the eAF (DADI)-PMS Newsletter is published and available on the EMA corporate website here.
The eAF-PMS Newsletter, published four times a year, provides pharmaceutical companies and the national competent authorities in the EU updates on the progress of the eAF and PMS products.
It also includes an overview of upcoming events and information on available resources.
For more insight on eAF, you are invited to register to the upcoming events and consult the following resources:
Upcoming events:
- Human Variations eAF Q&A Clinics:
- Human Variations eAF public training:
Q&A documents:
- General Q&A Document (link), including Q&A's on PMS (Product Management Services);
- Training Q&A document (link) addressing questions raised by users during earlier eAF (DADI) trainings.
- Human Variations eAF go-live Q&A session (link).
- Human Variations eAF go-live training session (8 November 2022) (link).
Please send any questions to eSubProgofficer@ema.europa.eu
21-12-2022
Updated draft eCTD v4.0 implementation timeline for EU now available
An updated draft timeline for the implementation of eCTD v4.0 in the EU is now available on the eCTD v4.0 page here.
And
Updated PLM Portal eAF timeline now available
Please find an updated version of the PLM Portal eAF (DADI) Human Variations Forms timeline. The slide is now available on the PLM Portal Forum and on the PLM Portal eAF (DADI) web page.
And
Updated PLM Portal Guide to eAF (DADI) Registration is now available
An updated version of the Portal (eAF) guide to registration is now available on the PLM Portal Forum and on PLM Portal eAF (DADI) webpage. The guide aims to support the users of the eAF Portal in completing the registration steps before accessing the PLM Portal. This update includes several updates across the document.
For any issues or technical support on PLM Portal, please raise a ticket with the EMA Service Desk portal. Please share any questions with us via eSubProgofficer@ema.europa.eu.
13-12-2022
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.3 released to production on 12th December 2022 are now available on the PLM Portal Forum and on the PLM Portal eAF (DADI) web page.
And
Update on PLM Portal eAF (DADI) 2023 UAT and releases
Following the launch of the web-based Human Variation electronic Application Form (eAF) for Centrally Authorised Products (CAPs) on the
Product Lifecycle Management (PLM) Portal, users have accessed the PLM Portal to work on variation applications using the new web-form. This is a valuable step for users to familiarise themselves with the system as well as for the development team to gain more insights on features to improve and issues to fix.
The development team's priority now is addressing issues in the web form and stabilising it. Following this, work will continue adding the data and functionalities needed to fully support national, MRP and DCP variations application procedures and submissions.
EMA has therefore decided that the previously planned industry external User Acceptance Test (UAT) in January 2023 will be rescheduled to a date expected to be announced in the first half of 2023. The UAT will be scheduled when there are meaningful new features and capabilities to be tested.
To support stakeholder' planning for possible participation in the UAT and anticipate eventual transition to the use of the web-forms please note the following:
- The target window for introducing Nationally Authorised Medicinal Products (all NAPs, including MRP/DCP) to the variation application form is now Q2 2023;
- In addition, further capabilitiesneed to be introduced to make the variations eAF ready to start a formal transition period, the target window for release of these features is Q2-Q3 2023
- More precise time windows will be shared in the first half of 2023 with the aim of providing a minimum of a 2-month lead time before the UAT or a major release
For more insight on eAF, you can use the following resources:
Upcoming events:
- Human Variations eAF Q&A Clinics:
- Human Variations eAF public training:
- EMA Quarterly System Demo
- Q&A documents:
06-12-2022
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to the portal made in releases on 14th November 2022 and on 28th November 2022 are now available on the PLM Portal Forum and on the PLM Portal eAF (DADI) web page.
And
Note on production release on 14th November 2022 on the PLM Portal eAF
As part of EMA's regular biweekly release schedule, a minor release including number of bug fixes and other changes went live on 14 November 2022.
This release was not announced in advance on the PLM Portal Forum in the manner the production team would usually aim to communicate. The procedure for the announcements and information for releases - major and minor - will be reviewed to ensure their timely provision.
No release notes were shared at the time of this release, however, the Release notes have now been updated and they are now available in the PLM Portal Forum and on the eSubmission website.
For any issues or technical support with EMA's IT systems, please raise a ticket with the EMA Service Desk portal.
Please share any questions with us via eSubProgofficer@ema.europa.eu.
17-11-2022
Updated PLM Portal Guide to eAF (DADI) Registration is now available
An updated version of the Portal (eAF) guide to registration is now available on PLM Portal eAF (DADI) webpage. The guide aims to support the users of the eAF Portal in completing the registration steps before accessing the PLM Portal. This update includes further details on the use of Multi Factor Authentication (MFA).
Following the release, users of the Human Variations eAF should expect continual work on improvements in the form of regular releases. These will be a mix of bug fixes, scheduled improvements, improvements based on user acceptance testing (UAT) feedback or that may be identified by users after the release and new features, such as additional structured data fields.
For any issues or technical support with EMA's IT systems, please raise a ticket with the EMA Service Desk portal.
Please share any questions with us via eSubProgofficer@ema.europa.eu.
04-11-2022
Web-based Human Variations eAF (DADI) now live on the new Product Lifecycle Management Portal
EMA is pleased to announce the web-based Human Variations electronic application form (eAF) for Centrally Authorised Products (CAPs) is now available on the new Product Lifecycle Management (PLM) Portal. This is a big, first milestone in the ongoing journey to improve the eAFs and related processes. EMA has been collaborating with the UNICOM consortium to develop the human variation form and other web-based forms.
Following the release, users of the Human Variations eAF should expect continual work on improvements in the form of regular releases. These will be a mix of bug fixes, scheduled improvements, improvements based on user acceptance testing (UAT) feedback or that may be identified by users after the release and new features, such as additional structured data fields.
As a reminder: the use of the new web-based form is optional. This release will not trigger a transition period towards mandatory use. The formal transition period will start when nationally authorised products (NAPs) and features supporting use cases covered will be added to the form. The current interactive PDF eAF will remain available until the end of the transition period.
The new PLM Portal will in due course host all eAFs. In the future it will also host the data input user interface for product management service (PMS) data and the interface to provide data for for electronic Product Information (ePI). The PLM Portal will develop over time to inform and support you in the use of these services. One new feature launched on the PLM portal is a chatbot through which you can ask questions about the web-forms and get quick answers.
More information on upcoming events and resources available to help users to get familiar with the new form and the new PLM Portal is available here.
31-10-2022
Updated Product Lifecycle Management Portal Guide to (DADI) eAF Registration is now available
An updated version of the PLM Portal (eAF) guide to registration is now available on PLM Portal eAF (DADI).
The guide aims to support the users of the eAF Portal in completing the registration steps before accessing the PLM Portal. Most of these steps are independent from the PLM Portal eAF and correspond to those to obtain registration to use other European Medicines Agency (EMA) systems. Applicants are invited to register for the new PLM Portal with the support of this updated guide.
You will be able to sign into the portal after the go-live of the Human Variations electronic Application form (eAF) for CAPs.
For any issues or technical support with EMA's IT systems, please raise a ticket with the EMA Service Desk portal.
Please share any questions with us via eSubProgofficer@ema.europa.eu.
26-10-2022
eSubmission Gateway XML delivery file update for Raw Data submissions
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced a small change to the delivery file (addition of new submission type for 'Raw data submission' (human domain only). Further information on the Raw data pilot submissions can be found here. Please note that this new submission type should only be used when it has been previously agreed that the product is taking part in the pilot. The details on the changes are provided in the related release notes which are available here and the updated user guide is available here.
Previously created delivery files should continue to work, however, it is always recommended to clear the cookies and the cache to ensure that the system works correctly.
eCTD EU Module 1 Specification now open for review and all stakeholders are invited to submit change requests on the specification - deadline extended until 1st November 2022
The Human Harmonisation Group has decided to open the eCTD EU Module 1 Specification for review and all stakeholders are invited to review the list of submitted change requests and, to submit new change requests on the specification.
Please find a list of submitted change requests and the template to submit any new change requests to the current version of the eCTD EU M1 specification. The comments should be submitted via email to EUM1Spec@ema.europa.eu by end of the day 1st of November 2022.
14-10-2022
Updated version of the eAF v1.26.0.0 (human variation) now available
An updated version 1.26.0.0 of the human variation eAFs is now available here.
A single change has been implemented to emphasize the mandatory use of OMS for centralised procedure by updating the Declaration label in the Proposed section. There is a very limited impact to users of the forms. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human).
Please note that an updated EMA practical guidance on the eAF for Type IA and IB variations is now available and this updated version of the guide addresses the use of this important declaration.
It is strongly recommended to use this latest version (document properties date 3.10.2022).
Please note that there is no version number change.
Please note that the release notes are not published for this minor change at this time.
Web-based Human Variations eAF go-live details
EMA is pleased to inform that the web-based Human Variations electronic application form (eAF) for CAPs - often referred to by its former project name: DADI - will be available for use on 4 November 2022 on the new Product Lifecycle Management (PLM) Portal. EMA has been collaborating with the UNICOM consortium to develop the new web-form. To ensure full technical and business support services at go-live, release to the public is scheduled slightly later than the original target date of 31 October. This is due to European Holidays on November 1 and 2.
Following the release, users of the Human Variations eAF should expect continual work on improvements in the form of regular releases. These will be a mix of scheduled improvements, improvements based on user acceptance testing (UAT) feedback or that may be identified by users after the release and new features, such as additional structured data fields. Going live as scheduled and continually improving the form is the most effective route to having a high performing web-form in the shortest timeframe and it is in accordance with an Agile way of working.
As a reminder: this release will not trigger a transition period towards mandatory use. The formal transition period will start after the anticipated March 2023 release when nationally authorised products (NAPs) features will be also added to the form. The interactive PDF eAF will remain available until the end of the transition period.
The new PLM Portal will in due course host all web based eAFs, data input user interface for product management service (PMS) data and the interface to provide data for electronic Product Information (ePI). You can expect reference to Digital Application Dataset Integration (DADI) to be phased out over the coming weeks in favour of eAF and the PLM Portal.
We have the following resources to help you get familiar with the new form and the new environment:
- Pre go-live:
- Publication of the updated guides for portal registration and navigation (to be made available via the eSubmission website).
- Human Variations Form go-live Q&A session: 27 October 2022 (14:00-15:00), Registration link.
- Post go-live:
- Human Variations web-based eAF Form training session: 8 November 2022 (10:00-11:30), Registration link.
• Q&A clinics:
Please note an updated general Q&A document, including Q&A's on PMS, and a training Q&A document addressing questions raised during earlier eAF trainings are now available.
Please share any questions with us at eSubProgofficer@ema.europa.eu
27-09-2022
DADI Project Update
System demo: digital application dataset integration (DADI) and Product Management Service (PMS) - Live broadcast available
EMA has announced the 3rd public system demo of 2022. In this system demo, EMA will demonstrate developments of DADI project and the PMS service as well as other ongoing projects. The system demo will take place on Wednesday 28th September 2022 at 09:00-12:30 (CET). This demo will be presented via live broadcast to ensure that all interested colleagues are able to attend the session.
No registration is required to follow the live broadcast on EMA's website, available from the event page. Kindly note the session will also be recorded and made available through EMA Corporate Website.
Please share any questions on the above system demo with us at eSubProgofficer@ema.europa.eu.
Update of the DADI Q&A document
The DADI and PMS teams are pleased to announce that a joint DADI and PMS Q&A Document is now available here. Given their interdependencies, this updated version of Q&A document includes questions related to both DADI and PMS.
Human variations form updated timeline
Please find the September 2022 version of the DADI Human Variations Forms timeline, outlining the updated DADI BETA UAT timeframe. The UAT has started on 19th September and will end on the 30th September 2022.
Recordings and documentation from the DADI training webinars held on 2nd September and on 26 July 2022 and Q&A webinar on the go-live held on 12th July 2022 now available
The recording and the presentation from the DADI training webinar on 2nd September 2022 are now available and the recording from the training webinar held on 26th July 2022 is available here. The recording from the Q&A on go-live held on 12th July is also now available.
eCTD EU Module 1 Specification now open for review and all stakeholders are invited to submit change requests on the specification
The Human Harmonisation Group has decided to open the eCTD EU Module 1 Specification for review and all stakeholders are invited to review the list of submitted change requests and, to submit new change requests on the specification.
Please find a list of submitted change requests and the template to submit any new change requests to the current version of the eCTD EU M1 specification. The comments should be submitted via email to EUM1Spec@ema.europa.eu by end of the day 25th of October 2022.
02-08-2022
Questions and Answers document from the public webinar “DADI Q&A Webinar - Variations form for Human Medicinal Products Go-live” held on 12th July 2022
The Q&A document from the public webinar is now available.
27-07-2022
The 1st DADI newsletter has been published on the EMA website
The digital application dataset integration (DADI) newsletter, published four times a year, provides pharmaceutical companies and the national competent authorities (NCAs) in the EU updates on the progress of the DADI project.
You can find the first issue of the newsletter here.
Presentation from the public webinar “DADI Q&A Webinar - Variations form for Human Medicinal Products Go-live” held on 12th July 2022
The presentation from the public webinar is now available.
14-07-2022
DADI eAF Portal Guide to Registration now available
The Digital Application Dataset Integration (DADI) Network project team is pleased to announce the publication of the first version of the eAF Portal guide to registration on the DADI website.
The guide aims to support the users of the eAF Portal in completing the registration steps before accessing the platform. Most of these steps are independent from the eAF Portal and correspond to those to obtain registration to use other European Medicines Agency (EMA) systems, such as Management Services for Substances, Products, Organisation and Referentials (SPOR).
For any issues or technical support with EMA's IT systems, please raise a ticket with the EMA Service Desk portal.
Invitation to register to the DADI eAF Training and Q&A webinars
The Digital Application Dataset Integration (DADI) Network project will replace PDF electronic application forms (eAF) used for regulatory submissions with web-forms, making the future form-filling and submission-handling process more efficient.
The variations form for Human medicinal products will be the first form to be released by DADI in October 2022. To effectively support stakeholders ahead of the go-live date, EMA is pleased to announce two new public eAF training and Q&A webinars.
- 1st eAF training and Q&A webinar: 26 July 2022, 11:00-12:30 Central European Time (CET) - Registration link
- 2nd eAF training and Q&A webinar: 2 September 2022, 11:00-12:30 Central European Time (CET) - Registration link
These events are training webinars for industry and national competent authorities' stakeholders wishing to learn more about access management aspects related to the new portal and the procedure to fill in a web-based eAF at go-live.
During the sessions, a demonstration focused on access management and the User Interface will be performed in order to showcase how to register and access the Portal, how to fill in the web-based eAF, select products and export the forms.
Kindly note recordings from both training sessions will be made available after each event.
Please share your questions on the above webinar with us at eSubProgofficer@ema.europa.eu.
Recordings and documentation from the DADI public webinar held on 16th May and the System Demo held on 28th June 2022 now available
The recording from the DADI public webinar on go-live and the Quarterly system demo (Q2 2022) are now available here for the 16th May and here for the 28th of June 2022. Documentations for the webinars are also available here.
11-07-2022
eSubmission Gateway Web Client - upgraded Syncplicity portal now in production
The eSubmission Web Client portal has now been upgraded to Axway Syncplicity portal. Please access the new Web Client to send submissions to the EMA here.
All previously registered eSubmission Gateway Web Client users should have now received an email from Axway with a link to reset your password for the production Web Client. Please see the Guidance on how to reset your password.
07-07-2022
DADI Human Variations eAF User Acceptance Testing (UAT) - Call for Volunteers
In the context of the digitalisation of the Variations Application Form for Human Medicinal Products, the EMA is pleased to invite Applicants to participate in the UAT of the Digital Application Dataset Integration (DADI) Network project.
UAT timeline and scope
The DADI UAT is planned to be conducted between 5th and 16th September 2022. During that period, the production-like version of the electronic Application Forms (eAF) Portal will be open for testing. As recently communicated, please note that the scope of this UAT is limited to Centrally Authorised Products (CAPs). The UAT participants are expected to perform testing activities, by filling in electronic Application Forms as close as possible to real-life scenarios. Namely, this implies filling in an eAF for one or multiple CAPs, single scope or grouping of scopes from different variation procedure types (e.g., type IA or type IB), and to export the PDF output to be included in a submission.
More information and how to register can be here.
05-07-2022
eSubmission Gateway Web Client upgrade - Syncplicity go-live for production environment on this weekend (8th - 10th July 2022)
As previously published, the EMA is in the process of upgrading our current Axway Gateway solution.
Please note that migration of all users in the Production System will be done this weekend, starting from Friday, 8 July 2022, at 17:00 CEST until Sunday, 10 July 2022, at 18:00 CEST. Should this need to be postponed, an update will be published on this webpage.
Please note that registered production environment users will receive an email from Axway Syncplicity with a link to reset your password for the production environment. Please see the guidance on how to reset your password.
In the view of the update for Gateway to Gateway users only, we have requested you to make the required changes to update your firewall rules in line with the gateway connections document. If you use the Gateway-to-Gateway connection (not relevant to Web Client users), please implement the required changes by Friday, 8 July 2022, at 17:00 CEST to avoid any business disruption.
DADI Human Variations eAF revised go-live scope
EMA will launch the eAF variations web form in October 2022. This is a first release of the web-based variation form for human medicinal products that will be improved and expanded in subsequent releases in 2023. This progressive release model follows the Agile development model of the Agency.
The scope of the October 2022 go-live will be limited to Centrally Authorised Products (CAPs). EMA made this decision due to the complexity in synchronisation of the data between xEVMPD and PMS.
A subsequent release of the form in March 2023 will support all types of EU variation procedures (CAPs and NAPs).
More information is available here.
15-06-2022
eSubmission Gateway Web Client upgrade - External UAT for all XCOMP users now open
The issues relating to preparations for the external UAT have been solved and all eSubmission Web Client XCOMP users are now invited to start user acceptance testing (XCOMP environment). Please refer to the Guidance on how to submit using Syncplicity Web Client.
There is no need to register for the UAT. Please provide any feedback via the EMA Service Desk as soon as possible, but latest by Wednesday 22nd June 2022.
It is currently anticipated by Axway that the go-live of the new Web Client (Syncplicity) will be in take place in late June 2022. Date is yet to be confirmed. Before the go-live in production, the 'sun Java' which is only available in Internet Explorer is still required.
14-06-2022
A new version of the eSubmission Gateway XML delivery file is now available
An updated version of the eSubmission Gateway XML delivery file user interface is now available here. The new version provides an update of the framework for the delivery file user interface for both, the eSubmission Gateway delivery file UI and the PSUR Repository UI. The update is mainly technical, providing a slightly different look and feel.
Additionally, 2 new submission types; Companion Diagnostics Consultation and Follow-up Companion Diagnostics consultation have been added into the Gateway Delivery file UI human domain. No other functional changes have been introduced in the user interface.
10-06-2022
eSubmission Gateway Web Client upgrade
eSubmission Gateway Web Client upgrade - Migration of all (XCOMP) users to Syncplicity ongoing
The issues relating to preparations for the external UAT have been solved and it is now expected that the eSubmission Web Client users can start the user acceptance testing within the next couple of days (XCOMP environment). Please note that internal testing is still ongoing, and it is recommended that external testers do not start testing yet.
The migration of all existing XCOMP environment users to Syncplicity was done on Wednesday 8th of June 2022 - please note that registered XCOMP users have received an email from Axway Syncplicity with a link to reset your password for the XCOMP (test) environment. Please note that this resetting of the password has no impact in the password in production eSubmission Gateway Web Client. Please see the guidance on how to reset your password.
The details on how to send test submissions will be published as soon as the user guide becomes available once the migration activities have been finalised.
There is no need to register for the UAT, and the link to the system will be provided via this website.
It is currently anticipated by Axway that the go-live of the new Web Client (Syncplicity) will be in take place in late June. Date is yet to be confirmed. Until then, the 'sun Java' is still required (only available in IE).
eSubmission Gateway Delivery file UI - technical update go-live postponed until Tuesday 14th June 2022
The eSubmissions team has been working on an update of the framework for the delivery file user interface.
This updated user interface will be deployed in production on Tuesday 14th of June 2022.
08-06-2022
eSubmission Gateway Web Client upgrade
The EMA is in the process of upgrading our current Axway Gateway solution.
The Gateway tool provider Axway is currently facing some issues with the new tool, however it is expected that the testing by external users can start within next couple of days.
Details and updated user guide will be published to support testing.
eSubmission Gateway Delivery file UI - technical update go-live 10th June 2022
The eSubmissions team has been working on an update of the framework for the delivery file user interface. This is mainly a technical update only providing a slightly different look and feel.
Additionally, 2 new submission types; Companion Diagnostics Consultation and Follow-up Companion Diagnostics consultation have been added. There are no other functional changes to the MAH user interface.
This updated user interface will be deployed in production on 10th of June 2022.
24-05-2022
eSubmission Gateway Web Client upgrade
Update on the project status
The EMA is in the process of upgrading the current Axway Gateway solution.
The Gateway tool provider Axway is currently facing some issues with the tool and this has caused a delay in the start of the User Acceptance Testing (UAT). Once these Syncplicity issues are resolved, Axway will propose a new timeline for the UAT and a date will be announced for the go-live in production.
Update of IP addresses for Gateway to Gateway connections
As part of an upcoming eSubmission Gateway upgrade which affects the Gateway to Gateway submission, the EMA will be changing the IP addresses of the AS2 Gateway connections. To ensure smooth transition, all current eSubmission Gateway to Gateway users are advised to update firewall rules in line with the gateway connections document. No other configuration changes are required as part of this upgrade.
This IP address range change does not affect the Web Client users.
If you have any questions on the upgrade, contact us using the EMA service desk.
eSubmission Gateway Delivery file UI - technical update
The eSubmissions team has been working on an update of the framework for the delivery file user interface. This is mainly a technical update only providing a slightly different look and feel. Additionally, 2 new submission types; Companion Diagnostics Consultation and Follow-up Companion Diagnostics consultation have been added. There are no other functional changes to the MAH user interface.
The updated user interface will be deployed in production once the internal user acceptance testing has been finalised (exact date TBC).
An updated user guide and release notes will be published at the time of the go live.
21-04-2022
eSubmission Gateway Web Client upgrade - Migration of all (XCOMP) users to Syncplicity starting now
The preparations for the external UAT are almost finalised and it is now expected that the eSubmission Web Client users can start the user acceptance testing within the next couple of days (XCOMP environment). The link to the test system and details on how to send test submissions will be published as soon as they become available.
There is no need to register for the UAT, and the link to the system will be provided via this website.
The migration of all existing XCOMP environment users to Syncplicity starting today - please note that you will be receiving an email from Axway Syncplicity with a link to reset your password for the XCOMP (test) environment. This resetting of the password has no impact in the password in production eSubmission Gateway Web Client.
The vgateway (old XCOMP environment) is currently not accessible.
The new Web Client (Axway Syncplicity) will be implemented in production environment in May 2022 following successful user acceptance testing.
10-03-2022
eSubmission Gateway Web Client upgrade planned for Spring 2022
The EMA eSubmission team is pleased to announce that a project to upgrade the eSubmission Gateway Web Client is ongoing and the updated version of the Web Client (Axway Syncplicity) will be implemented in May 2022 subject to successful user acceptance testing planned to take place in April 2022.
This upgrade will result in need for an update of the user passwords to allow access to the updated system. Further details on the participation in the UAT and on the steps required from existing users will be published in advance of the change.
28-01-2022
A new version of the eSubmission Gateway XML delivery file is now available
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced a large number of changes to the delivery files for Veterinary submissions reflecting the requirements/changes from the VMP-Reg (e.g. addition of new submission types, removal of submission types/submission units that are no longer relevant, update of available business units in relation to submission types for which no 'new applications' can be made once the new legislation is in force and addition of new values for Submission format dropdown menu).
Users should note that the delivery files created prior to the new release will not work after the go live.
18-01-2022
A new version of the eSubmission Gateway XML delivery file is now available
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced a small change to the delivery file (addition of new submission description 'Raw data pilot submission' for MAA 'additional information' (human domain only). Further information on the Raw data pilot submissions will be published soon and this new submission description should only be used when it has been previously agreed that the product is taking part in the pilot.
22-12-2021
Updated EU Harmonised technical eCTD guidance now available
An updated version of the EU Harmonised technical eCTD guidance and the related release notes are now available here. The updated guidance enters into force 1st February 2022.
And
Final, updated Veterinary eSubmission Guideline and other relevant veterinary eSubmission documents are now available
An updated version of the Veterinary eSubmission Guideline and other relevant veterinary eSubmission documents updated to reflect the VMP-Reg are now available here.
And
The EMA is closed 23 December 2021 to 3 January 2022 inclusive.
The EMA IT service desk closes at 18:30 (CET) on 22 December 2021.
A skeleton service will be available on 23rd, 27th, 28th, 29th, 30th and 31st (half day) December 2021, as well as on 3rd January 2022.
Please note that if the queries are complex or require specific expertise they may not be resolved until the service desk returns to normal service on 4 January 2022.
There will be no regulatory procedural support available during the EMA office closure.
We would like to advise to send all planned in December submission in timely manner considering limited availability of IT service desk during upcoming Christmas holiday.
10-12-2021
Invitation to register to the Digital Application Dataset Integration (DADI) Network Project Webinars
The Digital Application Dataset Integration (DADI) Network Project to replace electronic application forms is pleased to announce it's first public webinars which will take place on 18 January 2022, h. 10:30-12:00 Central European Time (CET) and on 25 January 2022, h. 10:30-12:00 Central European Time (CET).
Read more details here.
01-12-2021
eAF v1.26.0.0 now available
New version 1.26.0.0 of all eAFs is now available on the eAF website. The forms for human applications (maa, variation and renewal) are ready for immediate use. The use of the v1.26.0.0 for human procedures becomes mandatory after a short one-month transitional period on 1st January 2022. The version v1.25.0.0 of the human forms has been removed from the eAF website, however users can continue to submit applications using this version until the end of December 2021.
The main change in this version of the forms for human use relate to the mandatory use of OMS for Centralised Procedure applications. This version of the forms removes the free text fields in the forms when EU authorisation/Centralised Procedure is selected. Additionally, a bug fix relating to Medical Device section in the variation form has been provided.
The forms for veterinary applications are available for applicants and MAHs to familiarise with the updated forms prior to the mandatory use of this version for veterinary submissions from 28th of January 2022. Please note that there will be no transitional period once these forms go-live on the 28th of January. The v1.25.0.0 cannot be used for any new procedures starting after 28th January 2022.
The main changes in this version of the veterinary forms (maa and variation) relate to the implementation of the Regulation (EU) 2019/6 for Veterinary Medicinal Products.
The version 1.26.0.0 cannot be used for procedures prior to 28th January 2022, however, in the view of significant changes in the forms it is strongly recommended that applicants will carefully review the form prior to the mandatory use deadline in order to identify and report any issues in the implementation and thus allowing an opportunity to fix any issues found prior to 28th January 2022.
The version 1.25.0.0 will remain available for use for veterinary procedures (maa and variation) until the mandatory use of v1.26.0.0.
More details can be found from the release notes and the technical documentation available here.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure. This also applies to the veterinary procedures which have started prior to 28th of January 2022, it is important to note that the version of the form must not be changed during an ongoing procedure.
For any issues with the eAFs, or any issues in relation to the VMP-Reg, please raise a call via the EMA service desk.
11-11-2021
Essential maintenance to PSUR and eSubmission Gateway
Please note that PSUR and eSubmission Gateway File Handler will be unavailable from Friday 12/11/2021 after 18:00, until Monday 15/11/2021 morning 8:00, due to essential maintenance. EMA gateway will, however, remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete.
If you have any questions, please contact the IT service desk.
26-10-2021
Updated draft technical documents for eAF v1.26.0.0 now available
Updated draft DES summary and draft XSD files for the Veterinary MAA and variation forms are now available on the eAF website here.
And
Updated renewal form and veterinary maa eAF v1.25.0.0 now available
Updated versions of the renewal form and the vet MAA form v1.25.0.0 are now available on the eAF website. There is no change in the version number. This is a bugfix release to rectify 2 minor technical bugs which do not affect all users. More details are available on the updated summary release notes available on the eAF website.
25-10-2021
Call for volunteers for testing eAF v1.26.0.0 veterinary variation and maa forms (reflecting the VMP-Reg)
The version 1.26.0.0 of the electronic Application Forms (eAFs) is planned for release on 1st December 2021 (for review only) and mandatory use from 28th of January 2022. The release v1.26.0.0 will provide a major change in both variation and maa forms for veterinary applications to align with the new Veterinary Regulation (VMP-Reg). These changes will be detailed in the release notes. User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
The testing by Industry and NCAs is planned to take place as follows:
- Industry: from Fri 29/10/21 to Tue 09/11/21
- NCAs*: from Wed 03/11/21 to Fri 12/11/21 (*Based on the eAFs received from Industry)
Please note that these dates are subject to change depending on the outcome of internal UAT. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu.
11-10-2021
Updated version of the Q&A on the Mandatory use of OMS for CAPs now available
An updated version of the Questions and Answers document to support applicants and Marketing Authorisation holders in the implementation of the mandatory use of OMS for CAPs can be found here.
Please note that a training webinar on OMS will be held on 21st of October 2021, where registered participants will have the opportunity to clarify any outstanding questions. The webinar will be recorded and made available after the event.
06-10-2021
Questions and Answers document on the Mandatory use of OMS for CAPs now available
The use of Organisational Maintenance Services (OMS) will become mandatory for Centrally Authorised Products (CAPs) from 1st of November 2021.
01-10-2021
eAF v1.25.0.0 now available
New version 1.25.0.0 of all four eAFs is now available for immediate use.
The main changes in this version of the forms relate to the implementation of the Medical Device Regulation Art 2(1) of Regulation (EU) 2017/745 and include other changes as implemented in the latest version of the NTA application forms.
The version 1.25.0.0 can be used immediately, and it strongly recommended that it will be used for applications for products containing medical devices as soon as possible, however, the one-month transitional period will run until end of October after which the use of version 1.25.0.0 of the forms will become mandatory.
The version 1.24.0.1 has now been removed from the eAF website however users can continue to submit applications using this version until the end of October 2021.
More details can be found from the release notes and a presentation on changes available here.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
For any issues with the eAFs, for example reporting missing scopes, please raise a call via the EMA service desk.
Updated release schedule for eAF now available
An updated release schedule for the electronic Application Forms (eAF) is now available here.
Mandatory use of OMS in eAFs from 1st of November 2021
The use of OMS in the eAFs will become mandatory from 1st of November 2021. Please note that a release to remove the free text fields in the human forms for Centralised Procedure (v1.26.0.0) is planned for release in early December 2021.
Version 1.26.0.0 will be mandatory for use for human domain from 1st of January 2022.
Please note that this version cannot be used for veterinary submissions prior to 28th January 2022. For veterinary domain, there will be no transitional period and the version 1.26.0.0 will become mandatory on the 28th January 2022. The previous version of the forms should be used for already ongoing procedures and applicants are reminded that the version of the form should not be changed during an ongoing procedure.
Updated PAM submission form now available
The PAM submission form has been updated to contain new fields (Product name and MA number) and the updated for v2.0.0.0 is now available here.
28-09-2021
Further extension to deadline for mandatory use of OMS for all CAP submissions
In order to allow applicants and MAHs to timely register in OMS, the deadline for mandatory use has been further extended and the mandatory use of OMS Organisation Management Service (OMS) for CAPs now starts on 1st of November 2021. Early registration of site(s)/organisations in OMS is encouraged.
The EMA would like to emphasise the importance of site registration in OMS before the regulatory submission. This will avoid any delay in the start of these applications as applicants will be requested to register the site(s)/organisations during validation and prior to the start of the procedure.
10-08-2021
DADI Project update now available
DADI project update is now available here. This update includes a timeline for the first forms to be released, a list of features of the human variation form and an updated questions & answers document.
02-08-2021
Updated release schedule for eAF now available
An updated release schedule for the electronic Application Forms (eAF) is now available here.
And
Call for volunteers for eAF v1.25.0.0 (reflecting the updated Medical Devices regulation)
The version 1.25.0.0 of the electronic Application Forms (eAFs) is planned for release at the end of September 2021 with a short 1-month transitional period and mandatory use from 1st of November 2021. The release v1.25.0.0 will provide a major change into the section 2.2.4 of the human MAA form as well as adding similar section in the human variation form. Additionally, there are other minor changes across all forms. These changes will be detailed in the release notes. User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
The testing by Industry and NCAs is planned to take place as follows:
- Industry: from Tue 24/08/21 to Wed 01/09/21
- NCAs*: from Mon 30/08/21 to Fri 03/09/21 (*Based on the eAFs received from Industry)
Please note that these dates are subject to change depending on the outcome of internal UAT. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu.
Further details on the release scope and the confirmed dates for the UAT will be provided soon.
An updated release milestone plan and release schedule for the electronic Application Forms (eAF) will be published shortly.
And
Updated technical documents for eAF v1.25.0.0 now available
Updated draft DES summary and draft XSD files for the forms are now available on the eAF website here.
22-07-2021
Extended deadline for mandatory use of OMS for all CAP submissions
The EMA would like to inform applicants and Marketing Authorisation Holders (MAHs) of human and veterinary medicinal products that as part of the development of the Substance, Product, Organisation and Referential (SPOR) data management services, and in anticipation to the upcoming Digital Application Dataset Integration (DADI) project, the registration in Organisation Management Service (OMS) will become mandatory for new sites and organisations to be registered for a Medicinal Product as part of any regulatory procedure submitted to the Agency. This includes but is not limited to pre-submission phase activities (eligibility requests, pre-submission meeting requests, change in contact person requests, ...), marketing authorisation applications, line extensions and variations (type IA, IB and II) and renewals.
In order to allow applicants and MAHs to timely register in OMS, the previously communicated deadline of 1 August 2021 is extended until the end of September 2021. Early registration of site(s)/organisations in OMS is encouraged.
In the future, registering the site(s)/organisations in OMS will also become mandatory for national procedures. The Agency will provide additional information in due time.
The EMA would like to emphasise the importance of site registration in OMS before the regulatory submission. This will avoid any delay in the start of these applications as applicants will be requested to register the site(s)/organisations during validation and prior to the start of the procedure.
16-07-2021
Updated draft version of the VNeeS specification v3.0 now available
An updated version (v3.0) of the draft guideline on the specifications for provision of an electronic submission (eSubmission) for veterinary medicinal products (Draft VNeeS guideline) and further information is now available here.
12-07-2021
Mandatory use of OMS in eAF for all submissions to EMA
The EMA would like to inform applicants and Marketing Authorisation Holders (MAHs) that as part of the development of the Substance, Product, Organisation and Referential (SPOR) data management services, and in anticipation to the upcoming Digital Application Dataset Integration (DADI) project, the registration in Organisation Management Service (OMS) will become mandatory from the 1st of August 2021 for new sites and organisations to be registered for a Medicinal Product as part of any regulatory procedure submitted to the Agency. This includes but is not limited to pre-submission phase activities (eligibility requests, pre-submission meeting requests, change in contact person requests, ...), marketing authorisation applications, line extensions and variations (type IA, IB and II) and renewals.
The EMA would like to emphasise the importance of site registration in OMS before the regulatory submission. This will avoid any delay in the start of these applications as applicants will be requested to register the site(s)/organisations during validation and prior to the start of the procedure.
17-06-2021
DADI Project Questions and Answers now available
The European Medicines Regulatory Network's Digital Application Dataset Integration (DADI) project has released a Questions and Answers (Q&A) document with further details about the purpose and scope of the project.
The project team aims to follow up in the coming month with further details, including key milestones, the full list of features and the first draft of the FHIR message.
More details and Q&A are available here
and
All EMA IT systems unavailable from 18 to 20 June 2021
EMA will be performing essential maintenance to components of our IT infrastructure during the weekend of 18 to 20 June. The work will start at 18:00 on Friday, 18 June, and finish by 23:59 on Sunday, 20 June. From 18:30 on Friday, 18 June to 18:30 on Saturday, 19 June, all EMA systems will be unavailable.
The EMA corporate website will remain available during this period but it may not be possible to access any other EMA-hosted website or online application.
The EMA product emergency hotline and phone number for notifying EMA of suspected quality defects or product recalls will operate as usual during this period.
21-04-2021
Planned maintenance of the eSubmission Gateway and the eSubmission Gateway Web client
(Production Axway Gateway) this weekend
The Production Axway Gateway (eSubmission Gateway and the Web Client) will be
unavailable from Friday 23/04 at 18:00 until Monday 26/04 at 06:00 due to essential maintenance.
For any further information, please contact Service Desk
and
Essential maintenance to SPOR data services - 24 April 2021
Essential maintenance is scheduled to be carried out to SPOR data services -
between 8:00-12:00 on Saturday 24 April. Applications consuming real-time RMS data (PSUR
Repository, MMS-e, EudraGMDP, EudraCT, EU PAS (ENCepp) and eAF Web Application) will be
affected, as well as internal or external access to SPOR portal. For any further information,
please contact Service Desk
15-04-2021
All EMA IT systems unavailable from 16 to 18 April 2021
All European Medicines Agency (EMA) information technology (IT) systems apart from the corporate website
(www.ema.europa.eu) will be intermittently unavailable
from 18:00 on Friday 16 April to 11:00 on Sunday 18 April 2021 (Central European Summer Time, CEST),
due to essential maintenance.
The EMA corporate website will remain available during this period but it
may not be possible to access any other EMA-hosted website or online application.
The EMA product emergency hotline and phone number for notifying EMA of suspected quality defects or product recalls will operate as usual during this period.
09-04-2021
Updated eCTD EU M1 specification now available
The eCTD EU Module 1 specification has been updated. The updated EU M1 spec v3.0.4 and the related release notes are available
here. There are no changes to the DTD and hence
this version enters into force as of date of publication and can be used immediately. It is recommended
to use the new version as soon as possible.
Please note that the release notes
provide practical information on the changes and it is strongly recommended to review the
release notes to fully understand the changes made in the specification.
25-03-2021
Digital Application Dataset Integration (DADI) project
The European Medicines Regulatory Network has launched a new telematics project called the Digital Application Dataset Integration project (DADI) to modernise and improve use of the EU electronic Application Forms (eAFs). DADI has been established as the successor to the Common European Single Submission Portal (CESSP) phase 1 project.
More details are available here.
11-03-2021
A new version of the eSubmission Gateway XML delivery file is now available
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced changes to the delivery file (addition of ‘nitrosamine related’ radio button for initial variation submissions (human domain only) and renaming the ‘Customer Reference’ field to ‘purchase order number’ for all relevant submission types and submission units (H&V).
The details on the changes are provided in the related release notes which are available here and the updated user guide is available here.
Users should note that the delivery files created prior to the new release will not work after the go live
08-03-2021
A new version of the eSubmission Gateway XML delivery file for veterinary changes is now available
An updated version of the eSubmission Gateway XML delivery file user interface for veterinary MRL and referral submissions is now available. This update has introduced changes to the delivery file (changes in the available submission units and mandatory use of customer number and Purchase Order number). The details on the changes are provided in the related release notes which are available here and the updated user guide is available here
Users should note that the delivery files created prior to the new release will not work after the go live
04-03-2021
A new version of the eSubmission Gateway XML delivery file for veterinary changes is planned for release in the evening of 8th March 2021
An updated version of the eSubmission Gateway XML delivery file user interface for veterinary MRL and referral submissions is planned for release in the evening of 8th March 2021. This update will introduce changes to the delivery file (changes in the available submission units and mandatory use of customer number and Purchase Order number).
Users should note that the delivery files created prior to the new release will not work after the go live.
04-03-2021
A new version of the eSubmission Gateway XML delivery file planned for release in the evening of 11th March 2021
An updated version of the eSubmission Gateway XML delivery file user interface is planned for release in the evening of 11th March 2021. This update will introduce changes to the delivery file (addition of nitrosamine related radio button for initial variation submissions and renaming the Customer Reference field to PO number for all relevant submission types and submission units.
Users should note that the delivery files created prior to the new release will not work after the go live.
08-02-2021
Updated version of the VNeeS specification now available
The Guideline on eSubmissions for Veterinary products has been updated to version 2.7.1, applicable immediately. This minor change is Brexit related change in the list of country codes in table 4.
This change has no impact on the validation rules and the current VNeeS Checker tool (v2.6) remains unchanged.
16-01-2021
Mandatory use of eAF v1.24.0.1
It is now mandatory to use the version 1.24.0.1 of all four eAFs.
The term United Kingdom has been removed from the EU/EEA country grouping on 1st of January 2021 for selection in any version of the forms (in the fields where EU/EEA countries are listed).
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
For any issues with the eAFs, for example reporting missing scopes, please raise a call via the EMA service desk
17-12-2020
Following the deployment of the v1.17.3.0 of the PSUR Repository, the version release notes are now available here.
Previously created delivery files should continue to work, however, it is always recommended to clear the cookies and the cache to ensure that the system works correctly.
15-12-2020
eAF v1.24.0.1 now available
New version 1.24.0.1 of all four eAFs is now available to allow the users a short transitional period before the start of the mandatory use of the form. The changes in this version of the forms (v1.24.0.1) relate to the Member State, OMS and Country fields in all 4 forms and the changes are implemented due to the end of the transitional period following Brexit and in the view of the Northern Ireland protocol.
The changes, in summary, are either addition of a new ‘country’ United Kingdom (Northern Ireland) in the EU and EEA country lists and removal of United Kingdom from the EU and EEA country lists in RMS. Additionally, 3 new country ‘groupings’ have been created in RMS to either remove or add United Kingdom or United Kingdom (Northern Ireland) where these terms should or shouldn’t be displayed. Some minor amendments to which country grouping certain fields are pointing to have also been made.
The short transitional period will coincide with the transitional period with the previous version of the eAFs (v1.23.1.3 variation, renewal, MAA Vet)/1.23.1.4 MAA Human) which was initially planned to end on 15th of December 2020.
The users are now allowed to submit applications using version 1.23.1.3/1.23.1.4 until the end of 2020. The version 1.23.1.3/1.23.1.4 has been removed from the eAF website however, it does remain acceptable for submissions until the end of 2020.
The new Brexit related version can be used for procedures starting after 1.1.2021.
There will be a short transitional period for the use of previous version 1.24.0.0 until the 15th of January for those applications that do not require use of terms United Kingdom or United Kingdom (Northern Ireland).
From 1st of January 2021 term United Kingdom will be removed from EU/EEA country grouping and it will no longer be available for selection in any version of the forms (in the fields where EU/EEA countries are listed).
This version also includes a change in the Variation form, form validation rules for National Authorisation applications for grouping of a single variation scopes for multiple products.
More details can be found from the release notes and a presentation on changes available here.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
For any issues with the eAFs, for example reporting missing scopes, please raise a call via the EMA service desk.
New version of the PSUR repository is now available
A new version of the PSUR Repository (industry and NCA user interfaces) is now available. Minor changes have been introduced to both industry and NCA user interfaces due to the end of the Brexit transitional period on 31st of December.
The changes relate to use of terms United Kingdom (Northern Ireland) and new country code XI for UK(NI) in the product selection tables in the industry user interface.
Previously created delivery files should continue to work, however, it is always recommended to clear the cookies and the cache to ensure that the system works correctly.
03-12-2020
eAF issues with webservices fixed
The issue affecting the ‘Substance Type’ field in both, human and veterinary MAA forms has now been fixed and all the previously available substance types are
now again available.
01-12-2020
eAF issues with webservices - Update
An issue relating to the eAF webservice update which affected availability of terms in the ‘Pharmaceutical Form’ field in all 4 forms has been fixed (Monday 30th November). All pharmaceutical forms should now be again in the eAFs. Please note that as this issue affected the webservices to the form, not the forms themselves so there is no need to change the form, just ‘update lists’ if the terms are not yet visible.
Additionally, another issue affecting the ‘Substance Type’ field in both, human and veterinary MAA forms has been detected and we are working on a further fix to solve this issue as soon as possible.
And
The VNeeS Q&A relating to eSubmission for Veterinary Applications has been updated and is available here.
26-11-2020
eAF issue with webservices
An issue relating to the eAF webservice update has affected availability of terms in the ‘Pharmaceutical Form’ field in all 4 forms. We have released a fix that has partially solved the issue; however, we are aware that there are still many combined pharmaceutical forms that are currently unavailable in the eAFs. Please note that as this issue affects the webservices to the form, not the form itself, also previous versions of the form are affected. We are working on a further fix to solve this issue as soon as possible.
For any issues with the eAFs, please raise a call via the EMA service desk.
19-11-2020
A new version of the eSubmission Gateway XML delivery file is now available
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced multiple changes ranging from new fields to changing previously optional fields to mandatory fields and a major change for Paediatric submissions by completely transforming the delivery files for these types of submissions as we are preparing to extend the Common Repository to contain Paediatric submissions.
Details on the changes are described in the release specific presentations, the updated user guide and the updated release notes which can be found here and specific details relating to the Paediatric delivery files can be found here.
Users should note that the delivery files created prior to the new release will not work after the go live.
27-10-2020
eAF v1.24.0.0 important note to users
An updated version of the variation eAF v1.24.0.0 is now available on the eAF website. This release which does not change the form version number, provides a fix to the Adobe backwards compatibility issue and it should solve the issues some users have experienced with the scope selection dropdown list in section 3. This version also includes a change in the form validation rules for grouping of Type IA or Type IAIN variations.
Please ensure that upon opening the eAF you ‘trust’ the form by clicking the small exclamation mark at the top of the left-hand adobe pane and then selecting the trust option from the yellow banner which will open across the top of the forms. The drop-down menu’s will not work if the form has not been trusted.

The Presentations for applicants and NCAs on the release v1.24.0.0 have been updated and are available here.
For any issues with the eAFs, for example reporting missing scopes, please raise a call via the EMA service desk. |
05-10-2020
The Guideline on eSubmission for Veterinary products - version 2.7 entered into force on 01 October 2020.
29-09-2020
The EU Change Control Process
The EU Change Control Process for Veterinary has been updated and can be found here.
15-09-2020
eAF v1.24.0.0 now available
Version 1.24.0.0 of all four electronic Application Forms (eAFs) is now available. The release v1.24.0.0 provides a major change in section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation). Some defects have been fixed in MAA human, MAA vet and renewal (H&V forms.
The new version is implemented with 3 months transitional period. Mandatory use of v1.24.0.0 will start on 16th December 2020.
More details can be found from the release notes and the updated Practical user guide for electronic Application Forms as well as the presentation on changes available here.
Please note that due to the major change in the variation form, data imports from older versions of the variation form will not work.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
11-09-2020
eAF v1.24.0.0 will be released on 15th September 2020
Version 1.24.0.0 of all four electronic Application Forms (eAFs) will be made available on 15th September 2020 with a slightly longer, 3 months transitional period and mandatory use from December 2020. The release v1.24.0.0 will provide a major change in section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation). There has been minor defect fixes in the other 3 forms (MAA human, MAA vet and renewal (H&V).
We would like to thank all UAT participants for your efforts and very useful comments.
A slightly updated release milestone plan and release schedule for the electronic Application Forms (eAF) are now available.
04-09-2020
Issue with eSubmission Gateway and its infrastructure
For the last few days, the EMA Gateway and surrounding infrastructure experienced an unplanned outage.
This has resulted in delays in acknowledgment communication as well as internal delays in submissions distribution.
The service is now available but we request you do not resubmit packages as the process of sending acknowledgments is experiencing delays.
Thank you for your patience and understanding.
20-07-2020
eAF v1.24.0.0 - Updated UAT details, updated release milestone plan and updated release schedule for eAF now available
An updated release milestone plan and release schedule for the electronic Application Forms (eAF) are now available.
Version 1.24.0.0 of all the 4 electronic Application Forms (eAFs) is planned for release in September 2020 with a 2 month transitional period, and mandatory use from November 2020.
The release v1.24.0.0 will provide a major change into the section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation).
Please note that both MAA forms, human and veterinary, have been added into this release following a discovery of a defect in the forms.
The User Acceptance Testing (UAT) to support the release of this next version of the forms started on Friday 17th of July 2020.
Due to the delay in starting testing, we are now testing in parallel with both user groups (Industry and NCAs) and the UAT will run until Tuesday 4th of August 2020.
If you have not yet registered, however, wish to participate in the UAT, please contact us by email to eSubprogofficer@ema.europa.eu
As of 1.2.2020, the UK is no longer an EU Member State. However, EU law still applies to the UK during the transition period.
17-07-2020
An updated version of the EU Harmonised Technical Guidance for ASMF Submissions in eCTD format is now available here.
15-07-2020
Essential maintenance to eSubmission applications starting 18:00 on Friday 17th 2020
Please note that eSubmission Delivery file Web User Interface, PSUR Repository, Common Repository and the Gateway File Handler will be unavailable from Friday, 17 July 2020 18:00 until 14:00hrs on Saturday, 18 July due to essential maintenance. The EMA gateway will, however, remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. We recommend that you submit your package early if you have a deadline coinciding with the downtime.
If you have any questions, please contact the IT Service Desk.
And
The updated VNeeS change request tracking table is now available on the Veterinary eSubmission page.
Weekend maintenance to eSubmission applications starting 18:00 on Friday 17th 2020
Users should be aware that due to essential maintenance work on EMA’s infrastructure, most of EMA’s computer applications, including most eSubmission systems will experience downtime between the hours of 18:00 on Friday, 17th July and 14:00 on Saturday, 18 July 2020.
Please note that if you have submission deadline for your procedure on Friday, 17th July or Saturday 18th July, you may wish to submit the package prior to the submission deadline to avoid the downtime.
If you have any questions, please contact the IT service desk.
07-07-2020
Computer application maintenance Saturday, 11 July 2020
Essential maintenance work will be carried out to all eSubmission applications as part of EMA’s computer application maintenance work this weekend. As a result, eSubmission systems will be intermittently unavailable between 10:00hrs and 13:00hrs of Saturday, 11 July 2020. If you have any questions, please contact the EMA Service Desk.
29-06-2020
There is a short delay in the start of the UAT for the eAF v1.24.0.0 – new dates will be published very soon. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu
And
An updated version of the User guide to XML delivery file creation is now available here.
24-06-2020
A new version of the eSubmission Gateway XML delivery file now available (medical devices)
An updated version of the eSubmission Gateway XML delivery file user interface is now available. The update has introduced a label change to reflect the new Medical Device legislation.
Users should note that the delivery files created prior to the new release deployed in the evening of 23th of June 2020 will not work after the deployment. It is strongly recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues after the deployment. The updated system release notes detailing the change are available here.
11-06-2020
eAF v1.24.0.0 release milestone plan and updated release schedule for eAF now available
An updated release milestone plan and release schedule for the electronic Application Forms (eAF) are now available here.
Version 1.24.0.0 of the variation and renewal electronic Application Forms (eAFs) is planned for release in September 2020 with a 2 months transitional period and mandatory use from November 2020. The release v1.24.0.0 will provide a major change into the section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation). User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
The testing by Industry and NCAs is planned to take place as follows:
- Industry: from Mon 29/06/20 to Fri 03/07/20
- NCAs*: from Mon 06/07/20 to Fri 10/07/20 (*Based on the eAFs received from Industry)
Please note that these dates are subject to change depending on the outcome of internal UAT. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu
Further details on the release scope and the confirmed dates for the UAT will be provided soon.
CESP Dataset Module (CESSP Phase 1 Project)
The CESP Dataset Module, previously called CESSP Phase 1 project delivering a replacement of the PDF format electronic Application Forms (eAFs) is currently on hold. Further details and communication will be provided soon.
09-06-2020
A new version of the PSUR Repository (industry and NCA user interfaces now available
An updated version (v1.17.0.0) of the PSUR Repository is now available. There are minor changes to both, the industry and the NCA user interface. There is new functionality for EMA users, and a number of new notifications to different user groups.
Users should note that the delivery files created prior to the new release, deployed in the evening of 9th of June 2020 should continue to work following the deployment of this new version. However, it is recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues. The updated user guides (MAH and NCA), the PSUR Repository Training Guide for MAHs and NCAs and the system release notes detailing the changes are now available here.
And
Essential maintenance to computer application on Tuesday evening
eSubmissions Web User Interface (to create the delivery file) will be unavailable between 18:00-20:00hrs (CEST) on Tuesday, 9 June 2020.However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the IT service desk.
03-06-2020
Computer application maintenance – Sunday 7 June 2020
Essential maintenance work will be carried to eSubmission applications as part of EMA’s computer application maintenance work this weekend. As a result, eSubmission applications will be intermittently unavailable to users between 13:00 – 17:00hrs on Sunday, 7 June 2020.
29-05-2020
Please note that there has been an issue with the eSubmission Gateway submissions sent between Thursday 28th 3pm CET until 10am CET Friday 29th of May due to database issues. All submissions sent during this time have failed and the users have received a failure acknowledgement in to their Gateway mailbox. We encourage eSubmission Gateway users to check the mailbox for any failure acks and to resubmit where necessary. If you require assistance, please contact EMA service desk.
28-05-2020
The CMDh Best Practice Guide on the use of the electronic Common Technical Document (eCTD) in the Mutual Recognition and Decentralised Procedures has been updated and is available on the eCTD page.
14-05-2020
Essential maintenance to Gateway File Handler on Monday evening
Please note that eSubmission Gateway File Handler will be unavailable between 18.00-20.00hrs on Monday, 18 May 2020 due to essential maintenance. The EMA gateway will, however, remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete.
11-05-2020
New versions of the Q&A on how to handle ongoing procedures in relation to mandatory eCTD format and Q&A on mandatory eCTD in National Procedures (NP) are now available here.
05-05-2020
A new version of the eSubmission Gateway XML delivery file now available (Covid-19)
An updated version of the eSubmission Gateway XML delivery file user interface is now available. The update has introduced a new radio button to indicate if the submission is relating to Covid-19. This new flag will help to prioritise and indicate urgency of the incoming COVID19 related submissions.
Users should note that the delivery files created prior to the new release deployed in the evening of 5th of May 2020 should continue to work following the deployment of this new version. However, it is recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues. The updated user guide and the system release notes detailing the changes are available here.
31-03-2020
In the view that many applicants and MAHs are now working from home due to the Covid-19 situation, we would like to remind the users of the eAF functionality which may facilitate working during exceptional circumstances with variable internet bandwidths.
The electronic Application Forms can be saved directly from the eSubmission website to the users local machine and most fields can be filled in off-line, without access to internet. Only those fields that require substance/term related searches, and the use of OMS for address details requires connection to the internet. Validation feature and most dropdown lists are loaded to the form at the time when the form is opened and can be used even when the user is not connected to the internet enabling most of the form to be filled in without internet connection.
The performance of the forms with regards to the speed is unfortunately not optimal, mainly due to the technology used and the multiple business rules built in to the form, and hence, the network is working hard on the replacement of the PDF format forms with a web-based forms. The new web based forms for human and veterinary MAAs are planned for release later this year (timelines subject to change due to Covid-19 situation).
The eAFs are submitted as a part of the dossier which, for centralised procedure applications, must be submitted via the eSubmissions Gateway. In case the users are experiencing issues with the eAFs or preparing and sending submissions via the eSubmission Gateway or Web Client, we strongly recommend raising a ticket with detailed description of the issue via the EMA service desk portal.
It is important to include as many details as possible of the issue and, for example attaching screenshots or the form in question to aid investigation for the reasons of the issue.
And
The updated VNeeS guideline version 2.7 is now available here and will enter into force on 1st October 2020.
27-03-2020
The EMA has launched a survey to gather information from stakeholders to aid future planning of the development of the Gateway for the Electronic Standards for the Transfer of Regulatory Information (ESTRI).
Considering the European Commission (EC) policy on Digital service infrastructures and the EC recommendation for adoption of the AS4 messaging protocol for electronic exchange of data and documents, we are reviewing the current use of AS1 and AS2 capabilities and the transition expectations on the use of AS4 capabilities.
Please note that the survey has been sent via email to all EU QPPVs who are registered in EudraVigilance and the questionnaire only concerns the users of the AS2 Gateway. Users of the eSubmission Gateway Web Client are not impacted by the potential change of the protocol.
If your organisation has not received the questionnaire, and you are using, or planning to use the Gateway for submissions to the EMA, please contact the us using the service desk. Please note that the deadline for the survey is 3rd of April 2020.
25-03-2020
Updated Guidance on paediatric submissions is now available here. Applicants are reminded that the EMA does not accept submissions that do not follow the published guidance, including the submission channel. EMA encourages applicants who are new to the eSubmission Gateway to submit their application well in advance of the intended deadline.
24-03-2020
Computer application maintenance weekend 28-29 March 2020
Essential maintenance work will be carried out to all Telematics applications as part of EMA’s computer application maintenance work during the last weekend in March. As a result, e-Submission systems will be unavailable to users between 18:30hrs on Friday, 27th March and 08:00hrs on Monday, 30th March 2020
13-03-2020
Essential maintenance to computer application on Monday morning
eSubmissions Web User Interface (to create the delivery file) will be unavailable between 07:00-09:00hrs (CET) on Monday, 16 March 2020.However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the IT Service Desk
11-03-2020
The milestones for the CESSP phase 1 project have been updated and can be found here.
27-02-2020
An updated version of the eSubmission Gateway XML delivery file user interface for Veterinary ASMF submissions is now available. This update introduces a manual entry for ASMF numbers that are not available from the dropdown list enabling all Veterinary ASMFs to be sent using the XML delivery files.
The updated User guide to XML delivery file creation, presentation on changes and the release notes are available here.
And
Essential maintenance to computer applications on Thursday
PSUR and eSubmissions Web User Interface (to create the delivery file) will be unavailable between18:00-21:00hrs (CEST) on Thursday, 27 February 2020. However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the IT Service Desk.
10-02-2020
Essential maintenance to computer applications tonight (10-02-2020)
PSUR and eSubmissions Web User Interface (to create the delivery file) will be unavailable between 18:00-20:00hrs (CET) on Monday, 10 February 2020. However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the IT Service Desk.
01-02-2020
As of 1.2.2020, the UK is no longer an EU Member State. However, EU law still applies to the UK during the transition period.
09-01-2020
Please note that from 1 January 2020, the Formatted Table Template will no longer be maintained by EMA.
The use of the XML delivery files is mandatory and the users are required to fill in all the submission attributes correctly through the eSubmission Delivery File UI since all attributes in the delivery file are used to support important searches in the Common Repository.
The delivery file must be attached to the submission when it is sent via the eSubmission Gateway or the Web Client.
The use of XML delivery files also supports the EMA internal processes by significantly reducing the time required for receiving, processing and validating incoming applications and it ensures continuous and immediate access to up-to-date dossiers for internal and network users.
Please note that below mentioned fields are crucial and must be filled in correctly:
- Customer reference (Purchase order information) for all relevant fee related submissions
- Related procedure number for all submissions that already have a procedure number assigned
- RMP version number where applicable
- Withdrawal information for consolidating sequence unit type.
20-12-2019
The EMA is closed 23 December 2019 to 3 January 2020 inclusive.
The EMA IT service desk closes at 18:30 (CET) on 20 December 2019. A skeleton service will be available on 23rd, 24th (half day), 27th (half day), 30th and 31st (half day) December 2019, as well as on 2nd and 3rd January 2020. Please note that if the queries are complex or require specific expertise they may not be resolved until the service desk returns to normal service on 6 January 2020.
There will be no regulatory procedural support available during the EMA office closure.
09-12-2019
Version 1.23.1.4 of the Human Marketing authorisation Application (MAA) electronic Application Form (eAF) is now available. The release v1.23.1.4 is a bug fix release to correct business rules implementation in sections 1.4 and 1.5. Further details are available in the release notes available here. This bug fix release does not affect the Data Exchange Standard (DES).
05-11-2019
New version of the eSubmission Gateway XML delivery file now available
An updated version of the eSubmission Gateway XML delivery file user interface is now available. The eSubmission Gateway update has introduced an additional submission unit for PAM submissions and a mandatory selection of yes/no to indicate if a change of MAH is included in a response submission for MAA human and vet. Additionally some minor defects have been fixed.
Users should note that the delivery files created using the eSubmission Gateway XML delivery file UI prior to the new release deployed in the evening of 4th of November 2019 should continue to work following the deployment of this new version. It is however, recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues. The updated system release notes detailing the changes are available here.
Updated annexes to the eSubmission roadmap published
The following updated Annexes to the eSubmission Roadmap have been published to reflect current status of the practical implementation steps. Annex 2 to the HMA eSubmission Roadmap on the Mandatory use of eCTD for all procedure types and Annex 3 to the HMA eSubmission Roadmap on the implementation of mandatory VNeeS format for Veterinary regulatory submissions. The roadmap and the annexes can be found here.
31-10-2019
Essential maintenance to computer application on Monday evening
The eSubmissions Web User Interface (to create the delivery file) will be unavailable between 17:30-18:30hrs (CET) on Monday, 4 November 2019.
However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the IT Service Desk."
10-10-2019
Version 1.23.1.3 of the 4 electronic Application Forms (eAF) is now available. The release v1.23.1.3 is a bug fix release to correct number of issues that affect the forms. Further details are available in the release notes available here. This bug fix release does not affect the Data Exchange Standard (DES).
09-10-2019
Formatted Table Template implementation in the XML delivery files – now live
A new version of the eSubmission Gateway XML delivery file user interface is now available.
Following this release the use of the Formatted Letter template will become obsolete as of 1st January 2020. This will concern all EMA Human and Veterinary submissions (including PSUSA procedures) and after this it will be optional for applicants to provide the Formatted template as a part of the Cover Letter in Module 1 of eCTD sequences or Part 1 of VNeeS dossier.
From 1 January 2020, the Formatted Letter Template will no longer be maintained by EMA. The document and references will be removed from EMA corporate website.
Users are required to fill in all the submissions attributes correctly through eSubmission Delivery File UI by creating an XML delivery file since all attributes in the XML delivery file are used to support important searches in Common Repository. The use of XML delivery files will also support EMA internal processes by significantly reducing the time required for receiving, processing and validating incoming applications and it ensures continuous and immediate access to up-to-date dossiers.
Users should note that the delivery files created using the eSubmission Gateway XML delivery file UI prior to the new release will not work following the deployment of the new version. It is always recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues.
The changes are detailed in the release notes here.
Updated version of the PSUR Repository
Updated versions of the PSUR Repository Industry and NCA User Interfaces are now available. There are minor changes to the system and are detailed in the release notes here.
07-10-2019
Essential maintenance to computer applications on Wednesday 09-10-2019
PSUR and eSubmissions Web User Interface (to create the delivery file) will be unavailable between 07:00-09:00hrs (CEST) on Wednesday, 9
October 2019. However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the IT Service Desk.
01-10-2019
Version 1.23.1.3 of the 4 electronic Application Forms (eAF) will be available 10 October 2019. The release v1.23.1.3 is a bug fix release to correct a number of issues affecting the forms. More details on the fixes will be available in the release notes which will be published together with the updated forms. This hotfix release does not affect the Data Exchange Standard (DES).
Updated Release Milestone Plan for eAF and updated Release schedule for eAF and CESSP are available here.
25-09-2019
Formatted Table Template implementation in the XML delivery files
An updated version of the eSubmission Gateway XML delivery file user interface will be made available in early October 2019.
Following this release the use of the Formatted Letter Template will become obsolete as of 1st January 2020. This will concern all EMA Human and Veterinary submission (including PSUSA procedures) and after this it will be optional for applicants to provide the Formatted Template as a part of the Cover Letter in Module 1 of eCTD sequences or Part 1 of VNeeS dossier.
From 1 January 2020, the Formatted Letter Template will no longer be maintained by EMA and the document and references will be removed from EMA corporate website.
Users are required to fill in all the submissions attributes correctly through eSubmission Web UI by creating an XML delivery file, since all attributes in the XML delivery file are used to support important searches in Common Repository. The use of XML delivery files will also support EMA internal processes by significantly reducing the time required for receiving, processing and validating incoming applications and it ensures continuous and immediate access to up-to-date dossiers.
Users should note that the delivery files created using the eSubmission Gateway XML delivery file UI prior to the new release will not work following the deployment of the new version. It is always recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues. The updated system release notes detailing the changes will be made available at the time of the release.
And
Updated version of the PSUR Repository
An updated version of the PSUR Repository Industry and NCA User Interfaces will also be made available in conjunction with the new release of the eSubmission Gateway Delivery file UI. There are very minor changes to the system and these changes will be detailed in the release notes which will be published at the time of the release.
16-09-2019
The ICH M8 Vendor Readiness Survey is now closed. More information on eCTD v4.0 can be found here.
And
Weekend maintenance to most EMA computer applications during the weekend of 21st – 22nd September 2019.
Users should be aware that due to essential maintenance work on Oracle databases, most of EMA’s computer applications will experience downtime between the hours of 18.00 on Friday, 20 September and 06.00 on Monday, 23 September 2019.
If you have any questions, please contact the IT service desk.
10-09-2019
Please be informed that the VHG reached a decision regarding mandatory use of the PDF/A format in VNeeS, starting after the transition period when the CESP dataset module becomes mandatory for all submission types. The current planning in the new version of the eSubmission Roadmap that was recently adopted by HMA currently says that the CESP dataset module will be live for all procedures before the end of 2021 and then becomes mandatory 6 months after.
Relevant documents, i.e. VNeeS guideline and VNeeS checker tool will be updated and published 6 months before mandatory use of PDF/A.
09-08-2019
Vendor Readiness Survey
The ICH M8 EWG would like to assess the readiness of eCTD v4.0 Vendors and their development and/or readiness to deliver a production ready eCTD v4.0 solution. We request that the eCTD v4.0 Vendor Readiness Survey be completed by September 15, 2019.
More information about eCTD v4.0 including the EU IG package can be found here.
Updated reminder on the working documents provided within the eCTD submissions
As per the structure of the eCTD, the product information should be provided in PDF format within Module 1.1.3. However, occasionally it is necessary to provide, in addition to the PDF requirement, product information or the Module 2 documents in Word format. These files should be provided outside of the eCTD structure, in a separate folder called xxxx-workingdocuments on the same submission zip package as the eCTD.
For PMF certification submissions the ePMF should be provided within the working documents folder. This folder should be called “xxxx-workingdocuments” as for all other documents.
Other documents that can be provided as working documents outside of the eCTD structure include for example the RMP, (validation) checklists or any document specifically requested to be provided as a working document by the Health Authorities.
Please note: the Agency strongly recommends that applicants only include as part of the working documents folder applicable documents. Providing additional documents e.g. entire modules will cause delays in processing the application.
Please note: the eCTD validation report should not be included in the working documents folder. More information on the working documents can be found from the Harmonised Technical Guidance for eCTD Submissions in the EU.
22-07-2019
Essential maintenance to computer applications
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between between 19:00hrs (CEST) and 20:00hrs (CEST) on Tuesday, 23 July 2019. Applications consuming real-time RMS data (PSUR Repository, MMS-e, EudraGMDP, EudraCT, EU PAS (ENCepp), and eAF) will be affected intermittently during this time. Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository.
Search attribute MA authorisation type is also unavailable during this period. Please note that if you have submission deadline for non-EU single assessment PSUR on Friday, 26th July, you may wish to submit the PSUR prior to the submission deadline to avoid the downtime.
The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity.
19-07-2019
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between 18:00hrs (CEST) and 19:00hrs (CEST) on Friday, 19 July 2019 and between 21:00hrs (CEST) and 22:00hrs (CEST) on Sunday 21 July 2019. Applications consuming real-time RMS data (PSUR Repository, MMS-e, EudraGMDP, EudraCT, EU PAS (ENCepp), and eAF) will be affected intermittently during this time. Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository. Search attribute MA authorisation type is also unavailable during this period. Please note that if you have submission deadline for non-EU single assessment PSUR on Friday, 26th July, you may wish to submit the PSUR prior to the submission deadline to avoid the downtime.
The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity.
04-07-2019
The CESSP essentials document has been updated and can be found here
03-07-2019
Updated version 2.2 of the eSubmission roadmap published
An updated version of the eSubmission Roadmap has been published. The Roadmap has been updated to reflect achieved milestones and to include new and amended timelines for implementation of various telematics systems/standards. The updated Roadmap v2.2 and the visual representation of the steps are available here.
26-06-2019
Essential maintenance to computer applications
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between 08:00hrs(CEST) on Saturday, 29 June and 16:00hrs (CEST) on Sunday, 30 June 2019.
Applications consuming real-time RMS data (PSUR Repository, MMS-e, EudraGMDP, EudraCT, EU PAS (ENCepp), and eAF) will be affected intermittently during this time.
Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository. Search attribute MA authorisation type is also unavailable during this period.
Please note that if you have submission deadline for non-EU single assessment PSUR on Friday, 28th June, you may wish to submit the PSUR prior to the submission deadline to avoid the downtime.
The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations
and Locations are used (however manual input of data is allowed for these areas).
There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity.
05-06-2019
The milestones for the CESSP phase 1 project has been updated and can be found here.
03-06-2019
A webinar providing an update of the CESSP phase 1 project (converting the current eAFs MAA human and veterinary into web based forms) will be held on 5 June 2019. This webinar is aimed for Regulators and instructions on how to connect have been sent out by email to representatives of National Competent Authorities.
23-05-2019
The presentation and recording from the webinar providing an update to Industry about the CESSP phase 1 project held on 7th of May 2019 is now available here.
24-04-2019
A Q&A document on how to handle eCTD for ASMF submissions has been published on the eASMF page.
15-04-2019
A webinar providing an update of the CESSP phase 1 project (converting the current eAFs into web based forms) will be held on 7 May 2019 from 10:00 to 11:30 CET. This webinar is aimed for applicants (from pharmaceutical industry). A separate session aimed for regulators will be be organised in near future.
Please note that participation in this webinar is on first come first serve basis as the maximum capacity of the virtual meeting room is limited to 200 participants. If multiple users from your organisation are attending, please share the connection with your colleagues.
The webinar will be recorded and the recording will be made available on the CESSP webpage after the webinar.
How to login is available here.
10-04-2019
The eSubmission Gateway and Web Client online registration guidance has been updated with new contact information.
And
The applicants are reminded that eAFs should be edited and signed using Adobe Reader. Using Adobe Acrobat Pro may lead to issues when the regulators review the applications using Adobe Reader and this may lead to validation issues and delays in processing the applications. More information can be found from the eAF Q&A document.
And
The slides from the CESSP dataset module training held by the Network Training Centre are available here.
28-03-2019
The release notes for MAA Human and Veterinary electronic Application Forms (eAF) and the Renewal eAF have been updated to reflect new known issues. The release notes are available here.
26-03-2019
eAF dropdown lists not working?
Users are reminded that the eAFs will need to be ‘trusted’ in order for the dropdown lists to work. To ‘trust’ the forms, please click the exclamation mark on the top of the left hand pane and select ‘options’ to open the trust options. Select ‘trust this document always’ to proceed.

18-03-2019
A minor update has been made to the eAF – Questions & Answers document (Questions 9, 13 and 29). The updated version is available here.
15-03-2019
Version 1.23.1.2 of the 4 electronic Application Forms (eAF) is now available. This release v1.23.1.2 is an unplanned hotfix release to fix 3 issues that affected the functioning of the forms. More details on the fixes are available on the release notes. This hotfix release does not affect the Data Exchange Standard (DES).
This new version (v1.23.1.2) of the forms will fully replace the versions 1.23.1.0 and 1.23.1.1 after transitional period, on 4 April 2019.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
And
The final draft versions of the new XML schemas for the human and veterinary MAA forms that will be used in CESSP phase 1 are now available. The CESSP project is transforming the PDF format electronic Application Forms (eAFs) to interactive web based forms. Please provide your comments on the XSDs to cesspproject@hma.eu by 30th of April 2019.
And
The Network Training Centre is organising a webinar on utilising the upgraded XML schema of the initial application forms (eAF MAA human and Vet) to automate application data import into IT systems. The session will be held on 27th March 2019 at 10.30am CET. All relevant NCA staff is encouraged to register for the training on the NTC platform here.
28-02-2019
A minor update has been made to the Q&A on how to handle ongoing procedures in relation to mandatory eCTD format. The updated version is available here.
06-02-2019
The CESP dataset Module Milestones have been published and can be found here.
04-02-2019
Version 1.23.1.1 of the 4 electronic Application Forms (eAF) is now available. This release v1.23.1.1 is a bug fix release providing usability improvements and technical defect fixes. This bug fix release does not affect the Data Exchange Standard (DES).
This new version (v1.23.1.1) of the forms will fully replace the version 1.23.1.0 after transitional period, on 4 April 2019. The version of the form should not be changed during an ongoing procedure.
And
Delay in the Formatted Table Template implementation in the XML delivery files – please continue to use the Formatted Table Template until further notice (Human and Veterinary).
There is a delay in the launch of the Formatted Table Template fields in the eSubmission Gateway Delivery file User Interface (UI) due to interdependencies with other systems, extended testing and resource issues due to the move of the agency. We were therefore unable to discontinue the use of the Formatted Table Template as of 1st of February 2019 as previously announced.
The release of the updated eSubmission Delivery file UI for the eSubmission Gateway has been further postponed until a future date. We will provide an update once the timeline for the delivery can be confirmed.
Please continue to provide the Formatted Table Template for Human and Veterinary submissions as a part of the Cover Letter in Module 1 of eCTD submission sequences or Part 1 of VNeeS dossiers.
As usual, users are required to fill in all the submission attributes correctly through the Delivery file UI. The attributes in the XML delivery files are used to support important searches in the Common Repository and they support EMA internal processes. Please note that incorrect submissions may lead to procedural delays of incoming applications.
30-08-2018
Essential maintenance to eAF Web App
Electronic Application Form web application (eAF Web App) will be unavailable between 07:00 and 09:00 (UK time) on Friday, 31 August 2018 due to essential maintenance
29-08-2018
Human domain
The new eCTD validation criteria (v7.1) will come into force by 1 September 2018. There is no transition period. See this and further eSubmission information on the eSubmission webpage – eCTD v.3.2.
In addition, the NeeS validation criteria (v4.3) are updated as well. However, in line with the eSubmission Roadmap, they should now be applicable for National Procedures only, and normally only for other NP submissions than new MAA.
Veterinary domain
Guideline on eSubmissions for Veterinary products – version 2.6 entering into force on 1 of September 2018. Please see relevant documents on the Veterinary webpage.
Commission Implementing Regulation (EU) 2017/12 of 6 January 2017 regarding the form and content of the applications and requests for the establishment of maximum residue limits in accordance with Regulation (EC) No 470/2009 of the European Parliament and of the Council came into force on 27 January 2017.
With VNeeS version 2.6, the structure of the electronic dossier has been brought in line with structure described in the Commission Implementing Regulation.
For further information, please consult the VNeeS guideline or the VNeeS Q&A relating to eSubmission for Veterinary.
And
Reminder concerning the Common Repository for EMA coordinated procedures for Human Medicinal Products: applicants are reminded that from 1st of September for EMA coordinated procedures involving both Centrally and Nationally Authorised products, submission should be done only once, via the EMA eSubmission Gateway. No additional submission should be done via CESP.
The Dossier Requirements for referral, NAP and ancillary medicinal substances in medical devices document will be updated soon.
23-08-2018
Essential maintenance to computer applications
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between 08:00 and 18:00hrs (UK time) on Saturday, 25 August 2018. Applications consuming real-time RMS data (PSUR Repository, MMS-e, EudraGMDP, EudraCT, EU PAS (ENCepp), and eAF) will be affected during this time. Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository. Search attribute MA authorisation type is also unavailable during this period.
The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity
17-08-2018
Reminder concerning the Common Repository for Veterinary medicinal products: applicants are reminded that for procedures involving only Centrally Authorised products, submission should be done only once, via the EMA eSubmission Gateway. No additional submission should be done via CESP, CD or DVD, as National Competent Authorities have now access to the Common repository. For further details, please consult the Dossier requirements for submission of marketing authorisation and maximum residue limit (MRL) applications to the European Medicines Agency and to members of the Committee for Medicinal Products for Veterinary use (CVMP) (EMA/466102/2007)
13-07-2018
New versions of the 4 electronic Application Forms (eAF v. 1.23) and the related release notes are now available here.
This release includes further integration with OMS for organisation data in proposed and present part of Variation form and other NTA changes. This new version, 1.23 of the forms can be used as of today (13th July 2018) and will fully replace the version 1.22.0.1 after transitional period, on 15 October 2018. The version of the form should not be changed during an ongoing procedure.
09-07-2018
An updated version of the eSubmission Gateway XML delivery file user interface is now available. The eSubmission Gateway update will introduce an additional business rule to help users correctly select PASS 107 procedures types. Also,a Purchase Order number attribute for PASS 107 procedure was introduced.
This release of the eSubmission Gateway delivery file UI also fixes the known issue related to Veterinary submissions, users can now select from a predefined list of procedure numbers to reference their ASMF submissions. This is aligning with how Human procedure numbers can be linked to ASMF submission.
Users should note that the delivery files created using the eSubmission Gateway XML delivery file UI prior to the new release on 6st of July 2018 should work following the deployment of the new version. It is however, recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues. The updated system release notes detailing the changes are available here.
05-07-2018
Essential maintenance to computer applications on Friday
Common Repository, PSUR, Gateway File Handler and eSubmissions Web User Interface (to create the delivery file) will be unavailable between 07:00 and 09:00 on Friday, 6 July 2018.However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the IT Service Desk.
02-07-2018
eSubmission CMB and CMB documents have been renamed eSubmission expert group and eSubmission expert group documents. The eSubmission CMB changed its name in June 2018 to better reflect its current status and tasks.
29-06-2018
The document “Exceptions to the VNeeS format” has been updated. Please see the link in the section “Current Guidance” on the Veterinary eSubmission page.
22-06-2018
Updated documentation related to CESSP Phase 1 - i.e. Q&A, CESSP essentials project information and UAT2 results summary - have been published.
04-06-2018
Mandatory use of the Common Repository for veterinary submissions
The use of the Common Repository by National Competent Authorities (NCAs) to retrieve veterinary submissions is now mandatory since the 1st of June 2018. Please see further details in the Dossier requirements for submission of marketing authorisation and maximum residue limit (MRL) applications to the European Medicines Agency and to members of the Committee for Medicinal Products for Veterinary use (CVMP) (EMA/466102/2007)
And
The Common Repository has been extended to include EMA coordinated procedures from 8 March 2018. The use of the Common Repository for EMA coordinated procedures will become mandatory on 1st September 2018.
There should be no EMA coordinated procedures submissions sent directly to any NCAs from 1st of September 2018 as these will be considered to have been delivered to the NCAs. Please see the Statement of Intent on the mandatory use of Common Repository for EMA coordinated submissions for human medicinal products.
01-06-2018
Version 1.23 of the electronic Application Forms (eAFs) will become available on 13 July 2018. The release v1.23 will provide further integration with OMS from SPOR (Organisational/address data) and NTA changes,
User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
- The testing by Industry and NCAs will take place
- Industry: from Mon 11/06/18 to Fri 18/06/18
- NCAs*: from Mon 19/06/18 to Fri 22/06/18 (*Based on the eAFs received from Industry)
If you wish to participate, please register by email with eSubprogofficer@ema.europa.eu. A feedback form for consolidated comments will be provided to you following your registration. Please follow Summary of changes, document which shows clearly before and after areas and changes to eAF that focus on test scenarios for current UAT. UAT version of Summary of changes will be sent via email with UAT package. Final version will be published on website later (13 July 2018).
31-05-2018
Updated annexes to the eSubmission roadmap published
The following updated Annexes to the eSubmission Roadmap have been published to reflect further details on the practical implementation steps. Annex 3 to the HMA eSubmission Roadmap on the implementation of mandatory VNeeS format for Veterinary regulatory submissions, Annex 4 to the HMA eSubmission Roadmap on the replacement of the current PDF electronic application forms (eAFs) with the CESP Application Dataset Management Module (CESP Dataset Module) and Annex 5 to the HMA eSubmission Roadmap on the mandatory use of the Common Repository for EMA led procedures can be found here.
16-05-2018
Essential maintenance to computer applications
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between 18:00-19:00hrs (UK time) on Thursday, 17 May 2018. Applications consuming real-time RMS data (PSUR Repository, MMS-e, EudraGMDP, EudraCT, EU PAS (ENCepp), and eAF) will be affected during this time. Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository. Search attribute MA authorisation type is also unavailable during this period.
The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organizations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity
15-05-2018
The updated Annexes to the Roadmap are being published as they become available to reflect further details on the practical implementation steps. Annex 1 to the HMA eSubmission Roadmap on the implementation of eCTD version 4.0 and Annex 2 to the HMA eSubmission Roadmap on the implementation of mandatory use of eCTD format for Human regulatory submissions can be found here.
And
The updated DES documents and XSD files are now available for all 4 forms under the Technical Documents section. This is in preparation for the scheduled go live of eAF Version 1.23.
17-04-2018
Essential maintenance to computer applications on Wednesday 18 April 2008
The Gateway File Handler Registration module will be unavailable between 07:00 and 08:00 on Wednesday, 18 April 2018.However, the EMA gateway will remain available for all communities throughout. If you have any questions, please contact the IT Service Desk.
16-04-2018
The guideline on eSubmissions for Veterinary products – version 2.6, related Validation checklist 2.6 and VNeeS Checker version 2.6 have now been published. They will enter into force on 1 September 2018 - see links under section “Future Guidance” on the vet esubmission webpage.
The update of the guideline is related to alignment with the eSubmission roadmap and forthcoming CESP dataset module, clarification on upper-case file extension, update of CMDv reference guidance, addition of a pass/fail criterion related to hidden files, update of the MRL dossier structure.
13-04-2018
The eAF guidance for how to request new substances and terms has been updated. Please refer to the eAF Term Request Form section here.
12-04-2018
Updated version 2.1 of the eSubmission roadmap published
The updated version 2.1 of the eSubmission roadmap has been endorsed by HMA at their February meeting and has now been published here.
09-04-2018
Stepwise implementation towards mandatory use of the Common Repository for veterinary submissions
The use of the Common Repository by National Competent Authorities (NCAs) to retrieve veterinary submissions will be mandatory from 1st June 2018. Until this time, a transition period is ongoing, whereby the use of the Common Repository is being implemented in a stepwise approach, as indicated in the Dossier requirements for submission of marketing authorisation and maximum residue limit (MRL) applications to the European Medicines Agency and to members of the Committee for Medicinal Products for Veterinary use (CVMP) (EMA/466102/2007)
15-03-2018
Essential maintenance to EMA computer applications affecting PSUR Repository and eSUB UI on Sunday, 18 March
PSUR Repository and eSUB UI will be affected by essential maintenance to EMA computer applications between 09:00 and 14:00hrs (UK time) on Sunday, 18 March 2018. During this time, the NCA user interface (PSUR Repository access to documents) and the User Interface (for generation of the XML delivery file) will be unavailable. The eSubmission Gateway will continue to be available throughout this period so MAHs can send PSUR submissions.
08-03-2018
An updated version of the eSubmission Gateway XML delivery file user interface is now available. The eSubmission Gateway update will introduce improvements and new fields to the user interface. This change is a further step towards integration of the XML delivery file and the Formatted Table Template; however this release does not affect yet the use of the Formatted Table Template.
Please note that the delivery files created prior to the new release should work following the deployment of the new versions. One of the new features will be introduction of Brexit Related procedure indicator.
Users can indicate if the change is a Brexit related procedure. This is applicable to initial submissions for the following submission types:
- Variations Type IA (H & V)
- Variations Type IAIN (H & V)
- Variations Type IB (H & V)
- Variations Type II (H & V)
- Transfer MA (H & V)
- Notification 61-3 (H only)
Common Repository (User Interface and API) is now extended to include the following types of submissions related to procedures for Human Medicinal Products: Signal Detection, PASS 107 NAPs, Work share NAPs and Ancillary products for new submissions from the 8th of March 2018 onwards. This will be followed by a transitional period of 6-9 months, during which all NCAs will have time to adapt their systems and business processes in order to switch to the sole use of the Common Repository. During the transitional period, NCAs can still receive submissions directly from applicants, but they will also have the opportunity to switch to the sole use of the Common Repository during this period as and when they are ready to do so. Following the transitional period, the use of the Common Repository for above submissions will become mandatory for all NCAs. The date for the mandatory use is not yet decided.
07-03-2018
Essential maintenance to computer applications
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between 07:00 (UK time) on Saturday, 10 March and 18:00 (UK time) on Sunday, 11 March 2018. Applications consuming real-time RMS data (PSUR Repository, MMS-e, EudraGMDP, EudraCT, EU PAS (ENCepp), and eAF) will be affected during this time. Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository. Search attribute MA authorisation type is also unavailable during this period. Please note that if you have submission deadline for non-EU single assessment PSUR on Friday, 9 March, you may wish to submit the PSUR prior to the submission deadline to avoid the downtime.
The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organizations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity.
06-03-2018
The updated eCTD validation criteria v7.1 is now available here. The updated validation criteria will enter into force on 1st of September 2018.
The NeeS validation criteria v4.3 has been updated in-line with the updated eCTD validation criteria. The updated document is now available here. The updated validation criteria will enter into force on 1st of September 2018.
The EU Harmonised Technical Guidance for ASMF Submissions in eCTD format (version 2.1) has be edited in chapter 4 to clearly address rules on how to submit Letter of Access.
And
Essential maintenance to computer applications on Thursday 8 March 2018
Common Repository, PSUR, Gateway File Handler and eSubmissions Web User Interface (to create the delivery file) will be unavailable between 14:00 and 15:00 hrs (UK time) on Thursday, 8 March 2018.However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete.
23-02-2018
A new version of the Common Repository will be available in March 2018 extending the system to other EMA led EU procedures submissions.
Common Repository will extend and/or add new Cabinets to include the following types of submissions related to Human procedures:
- Signal Detection
- PASS 107 NAPs
- Work share NAPs
- Ancillary Products
19-02-2018
The use of the eSubmission Gateway and/or the Web Client become mandatory for all paediatric applications from 1 January 2018. To allow applicant to adapt to the new submission requirements, the EMA will allow a transition period until 15 March 2018. After this date, the European Medicines Agency (EMA) will no longer accept submissions by Eudralink.
16-02-2018
New versions of the 4 electronic Application Forms (eAF v. 1.22.0.1) and the related release notes are now available. This new version is a hotfix release v1.22.0.1 of the forms and can be used as of today, 16th February 2018. The version of the form should not be changed during an ongoing procedure.
15-02-2018
As of 15 February 2018 version 1.22 of the four electronic Application Forms (eAFs) are mandatory. Older versions of the forms will no longer be supported.
15-12-2017
New versions of the 4 electronic Application Forms (eAF v. 1.22) and the related release notes are now available here.
This release includes integration with OMS for organisation data and RMS for reference data.
OMS will provide standardised organisation and location data. (For information, eAF has been integrated with RMS since June 2017.)
This new version, 1.22 of the forms can be used as of today (15th December 2017) and will fully replace the version 1.21 after transitional period, on 15 February 2018.
The version of the form should not be changed during an ongoing procedure.
13-12-2017
Please see below the summary of post User Acceptance Testing for eAF v1.22 outcome.
In total 247 comments were received.
We have fixed 62 defects (remaining defects will be assessed and prioritised in future releases).
All submitted change requests will be analysed and prioritised.
| |
|
Change Requests |
Defects |
Defects Addressed |
| eAF |
MAA(Human) |
19 |
38 |
33 |
| |
MAA(Vet) |
2 |
6 |
5 |
| |
Renewal |
9 |
16 |
11 |
| |
Variation |
27 |
22 |
13 |
| |
|
57 |
82 |
62 |
08-12-2017
From 1 December 2017, the use of Common Repository for Referral submissions replaces the use of CESP and CD/DVD for sending Referral submissions to the National Competent Authorities (NCAs). All human referral submissions sent to the EMA will be considered delivered to all NCAs representatives and alternates.
Submissions for all referrals should only be made using the EMA eSubmission Gateway/Web Client.. Additional copies should not be submitted directly to the NCAs on CD/DVD or via CESP as this might lead to validation issues and cause delays. More information on the Common Repository can be found here.
Formatted Cover Letter Template - Survey
The use of the XML delivery files is now mandatory for all Human and Veterinary submissions via the eSubmission Gateway and the Web Client.
Release of XML delivery file was introduced in anticipation of future replacement and use of the Formatted Table Template that is currently provided as a part of the submission in cover letter.
We would like to know how existing business processes will be affected when this document is no longer required. The survey can be found here.
27-11-2017
Essential maintenance to computer applications
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between 18:00 (UK time) on Wednesday, 29 November and 24:00 (UK time) on Friday, 1 December 2017. Applications consuming real-time RMS data (PSUR Repository, MMS-e, EudraGMDP, EudraCT, EU PASS (ENCepp), and eAF) will be affected during Wednesday evening, Thursday and Friday. Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository. Search attribute MA authorisation type is also unavailable during this period. Please note that if you have submission deadline for non-EU single assessment PSUR on Friday, 1 December, you may wish to submit the PSUR prior to the submission deadline to avoid the downtime.
The eAF will be impacted only for the ATC code field (MAA human and vet and the renewal form). There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity.
22-11-2017
Common Repository, PSUR Repository, Gateway File Handler and the eSubmissions Web User Interface (to create XML delivery files) will be unavailable between 07:00 and 09:00 on Thursday, 23 November 2017.The EMA eSubmission Gateway and Gateway Web Client will remain available and any files submitted during that time will be queued for processing when the work is complete. If you have any questions, please contact the EMA IT service desk A new version of the Common Repository, extending the system to all EMA coordinated veterinary submissions is available from Thursday 23rd of November 2017. The new system can be accessed by all National Competent Authorities (NCAs) and committee members to search, view and download veterinary submissions.
The dossier requirements for veterinary submissions remain in place until the use of the system becomes mandatory in Q2 2018. Applicants should continue sending applications to EMA via the eSubmission Gateway and to the NCAs via CESP or CD/DVD as listed the dossier requirements document until it is announced by the EMA that the NCA is using the system exclusively.
The eSubmission Web User Interface for creation of the XML delivery files has been updated to include submission description (submission description – Responses to RSI) and updated values in submission format for veterinary PSUR submissions. A procedural change is also being introduced to the submission process for Worksharing (WS) and Internal Grouping (IG) procedures. An updated User Guide to XML delivery files will be made available here.
A webinar on the use of OMS and RMS data in eAF will be held on 28 November 2017 at 14:00-16:00 UK time. If you would like to participate in this webinar, please register by email to SPOR-Change-Liaisons@ema.europa.eu. Please note that participation in this webinar is on first come first serve basis as the maximum capacity of the virtual room is 200 participants. The webinar will be recorded and published on the eAF website.
And
eCTD EU Module 1 specification has been updated. The updated EU M1 spec v3.0.3 and the related release notes are available here. There are no changes to the DTD and hence this version enters into force as of today and can be used immediately. The users are recommended to use the new version as soon as possible.
The updated eCTD validation criteria v7.0 and related release notes are now available here. The updated validation criteria will enter into force on 1st of September 2018.
The NeeS validation criteria v4.2 have been updated in-line with the updated eCTD validation criteria. The updated document and the related release notes are now available here. The updated validation criteria will enter into force on 1st of September 2018.
A draft release schedule for 2018 the electronic Application Form is now available here.
User Acceptance Testing (UAT) started for the eAF v1.22 on Monday 13th November and will run until Friday 24th November 2017. The version 1.22 will integrate use of OMS (organisational data from SPOR programme) into all 4 forms as well as number of other user improvements. If you wish to take part in the testing, please register by email with eSubprogofficer@ema.europa.eu.
14-11-2017
Essential maintenance to computer applications on Wednesday
PSUR/eSubmissions Web User Interface (to create the delivery file) will be unavailable between 17:00-18:00 on Wednesday, 15 November 2017.However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete.
31-10-2017
An updated version of the Harmonised Technical Guidance for ASMF Submissions in eCTD format and the related Release notes are now available here. This version comes into force immediately.
Please note, that the EU eSubmission Roadmap and related Annex II include details on mandatory use of eCTD format for all submissions within EU procedures from January 2018. This is also applicable to ASMFs used for Marketing Authorisation Applications in all EU procedures.
25-10-2015
Updated versions of the Data Exchange Standard (DES) and XSD are now available on the eAF webpage for the next version of the electronic Application Forms (eAF).
The version 1.22.0.0 is planned to go live on 15th December 2017 following User Acceptance Testing (UAT) taking place in November 2017. If you wish to take part in the UAT, please register via email eSubProgOfficer@ema.europa.eu
The updated ‘CESSP Essentials’ presentation and a new CESSP Q&A document are now available on the CESSP webpage.
19-10-2017
Version 1.22 of the electronic Application Forms (eAFs) will become available on 15 December 2017. The release v1.22 will provide integration with OMS from SPOR (Organisational/address data), usability improvements and technical defect fixes. User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
- The testing by Industry and NCAs will take place
- Industry: from Mon 13/11/17 to Fri 17/11/17
- NCAs*: from Mon 20/11/17 to Fri 24/11/17 (*Based on the eAFs received from Industry)
If you wish to participate, please register by email with eSubprogofficer@ema.europa.eu. A feedback form for consolidated comments will be provided to you following your registration. A kick-off meeting is planned to take place on 13th November 2017 to explain the new features.
09-10-2017
Essential maintenance is scheduled to be carried out to SPOR data services: Referential Management Services (RMS) and Organisations Management Services (OMS) between 18:00 (UK time) on Thursday 12 October and 24:00 (UK time) on Monday, 16 October 2017. Applications consuming real-time RMS data (PSUR Repository, ECD Self registration, MMS-e, EudraGMDP, EudraCT, EU PASS (ENCepp), and eAF) will be affected during Thursday evening and Friday.
Should you have any questions please contact the EMA IT Service Desk.
The PSUR Repository will be impacted for industry users sending non-EU single assessment PSURs only and the NCA users using performing searches in the non-EU single assessment area of the PSUR Repository. Search attribute MA authorisation type is also unavailable during this period. Please note that if you have submission deadline for non-EU single assessment PSUR on Friday 13th October, you may wish to submit the PSUR prior to the submission deadline to avoid the downtime.
The eAF will be impacted only for the ATC code field (MAA human and vet and the renewal form). There is no impact to the Variation application form. All other dropdown menus for controlled terminology continue working during this maintenance activity.
05-10-2017
The EMA is launching an updated version of the EudraVigilance on 22 November 2017. In preparation for this update, there will be a downtime period for the Article 57 database (EXVDMP). During the scheduled downtime from 8th-21st of November 2017 it will not be possible to amend or add product/substance data in Article 57. This will impact the PSUR Repository as the product selection for the PSUR submissions is linked to Art. 57. It will not be possible to update/amend product data for the creation of the XML delivery files during this downtime period.
The EMA strongly recommends that MAHs, with a PSUR submission date falling during the scheduled downtime (8 to 21 November 2017), carefully review the list of products available through the PSUR Repository xml delivery file user interface to ensure availability of the products included in the relevant procedures and make plans to create the xml delivery file and submit their PSUR prior to the 8 November. Alternatively, if an earlier submission cannot be accommodated MAHs are encouraged to review the availability of products and correctness of the entries in the XML delivery file user interface. The MAHs should contact the EMA service desk to request a late submission ID in order to submit initial PSUSAs after the due date. There is no need to request late submission ID for subsequent submissions, such as responses.
This will also impact eAF use as the substance selection for the Initial MAA is linked to EXVDMP. It will not be possible to update substance data for the creation of eAF dataset during this downtime period. The EMA strongly recommends that MAHs, with a MAA date falling during the scheduled downtime (8 to 21 November 2017), carefully review relevant data and ensure that the required substances are available via the eAFs. More information is available from the EudraVigilance system downtime document.
28-09-2017
An updated version of the eSubmission Gateway XML delivery file user interface is now available and effective 29 September. This update introduces additional attributes to the veterinary domain in preparation for the extension of the Common Repository for Veterinary submissions. This release contains number of changes for veterinary users only, such as use of the ‘Submission description’ and selection of ‘procedure numbers’ for post-authorisation procedures where applicable. The updated User Guide, a short presentation with specific details on the changes and the updated release notes detailing the changes are available here.
26-09-2017
Essential maintenance to computer applications on Friday
eSubmissions Web User Interface (to create the delivery file) will be unavailable between 07:00 and 09:00 on Friday, 29 September 2017 However, the EMA gateway will remain available for all communities throughout and any files submitted during that time will be queued for processing when the work is complete.
The Common Repository will also be unavailable between 07:00 and 09:00 on Friday, 29 September 2017 due to essential maintenance.
22-09-2017
An updated version of the eSubmission Gateway Web Client will become available on Saturday 23rd of September 2017. The eSubmission Gateway Web Client update will introduce an updated user interface with some updated functionality. The user guide How to send submissions via the Web Client has been updated and is available here.
Romania is now using the Common Repository to receive Referral submissions. Please do not send any Referral submissions (including CAPs and NAPs) to Croatia, Estonia, Finland, Norway, Latvia and Romania, as these types of submissions are now retrieved via the Common Repository. Please see details on the Common Repository page.
The eCTD v4.0 working group would like to offer an opportunity for all eCTD tool vendors to discuss open points and ask questions on eCTD v4.0 EU Module 1 Implementation Guide. A face-to-face vendor meeting is planned to take place at BfArM in Bonn, Germany on 6th of October 2017. All vendors are invited, at their own expense to take part. All participants must register in advance, by 22nd September 2017 to eCTD4consultation@ema.europa.eu.
18-09-2017
Essential maintenance to EMA computer applications affecting PSUR Repository/eSUB UI/eSubmission Gateway on Saturday, 23 September
PSUR/eSubmissions Web User Interface (to create the delivery file) will be intermittently unavailable between 09:00 and 14:30 on Saturday, 23 September due to essential maintenance. The eSubmission Gateway will be also unavailable between 09:00 and 17:00 (UK time) due to essential maintenance.
15-09-2017
Croatia and Finland are also now using the Common Repository to receive Referral submissions. Please do not send any Referral submissions (including CAPs and NAPs) to Croatia, Estonia, Finland, Norway and Latvia, as these types of submissions are now retrieved via the Common Repository. More details on the Common Repository can be found on the Common Repository website.
13-09-2017
The Common Repository has been extended to include all human Referral submissions from 1st September 2017. The use of the Common Repository for human Referral submissions will become mandatory for all NCAs on 1st of December 2017. There should be no referral submissions directly to any NCAs from 1st of December 2017 as these will be considered to have been delivered to the NCAs. Please see the Statement of Intent on the mandatory use of Common Repository for human Referral submissions.
Estonia, Norway and Latvia are now using the Common Repository to receive Referral submissions. Please do not send any Referral submissions (including CAPs and NAPs) to Estonia, Norway or Latvia, as these types of submissions are now retrieved via the Common Repository. Please see details on the Common Repository page.
Applicants and MAHs are reminded that eCTD technical validation reports should not be included within the working documents folder when sending submissions to the EMA via eSubmission Gateway. The EMA does not require any eCTD validation reports as a part of a submission.
The use of the eAF versions 1.21.0.0 for variations and v1.21.0.1 for MAA human and vet and renewals is now mandatory since 1st of September 2017.
Please note that the all support calls relating to eSubmissions systems, such as eAF, must be sent via the EMA service portal. The email addresses ‘eAF@ema’ and ‘eCTD@ema’ are no longer in use and queries sent to these email addresses will not be processed.
01-09-2017
An updated version of the eSubmission Gateway XML delivery file user interface is now available. The eSubmission Gateway update will introduce an additional attribute ‘PAM code’ in to the XML delivery file. More information on the use of the PAM submission form can be found from the Post-Authorisation Guidance on PAMs; Question: How should I structure my PAM submission dossier? Please note: the PAM submission form should be provided within the eCTD submission package in the same folder as the cover letter (EU M1 section 1.0).
This release of the eSubmission Gateway delivery file UI also fixes the known issue related to submission description ‘Responses to RSI’. The updated User Guide describing the use of the PAM code as well as the updated system release notes detailing the changes are available here.
Users should note that the delivery files created using the eSubmission Gateway XML delivery file UI prior to the new release on 1st of September 2017 should work following the deployment of the new version. It is however, recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues.
The Common Repository has been extended to contain all human Referral submissions. The use of the Common Repository for referral submissions will become mandatory after a transitional period. The date for the mandatory use for Referral submissions by all NCAs will be announced shortly. The MAHs are advised to review the Dossier Requirements for referral, NAP and ancillary medicinal substances in medical devices document.
Latvia is now using the Common Repository to receive Referral submissions, in addition to all CAP submissions, from 1 September 2017. Please do not send any Referral submissions (including CAPs and NAPs) to Latvia as these are now retrieved via the Common Repository. Please see details on the Common Repository page.
31-08-2017
Essential maintenance affecting eSUB Web User Interface on Friday afternoon
eSubmissions Web User Interface (to create the delivery file) will be unavailable between 15:30 and 16:00 on Friday, 1 September 2017. However, Axway gateway will be available for all communities and any files submitted during that time will be put in a queue for processing when the deployments will be completed. If you have any questions, please contact the IT Service Desk.
25-08-2017
Essential maintenance to computer applications on Wednesday morning
Common Repository will be unavailable between 07:00 and 09:00 on Wednesday, 30 August 2017 due to essential maintenance. If you have any questions, please contact the IT Service Desk.
23-08-2017
The VNeeS Q&A relating to eSubmission for Veterinary Applications has been updated and can be found here.
General FAQ relating to PDF/A has been published on the Veterinary eSubmission page.
15-8-2017
The updated version of the eCTD v4.0 EU M1 Implementation Guide was released for public consultation on 20th May 2017. The commenting period has been extended to allow sufficient time for thorough review by pharmaceutical companies, vendors of eCTD building and reviewing tools and competent authorities. The new date to submit your comments is 20th of October 2017. Please provide your comments using the comment template to eCTD4consultation@ema.europa.eu.
The eCTD v4.0 working group would like to offer an opportunity for all eCTD tool vendors to discuss open points and ask questions on eCTD v4.0 EU Module 1 Implementation Guide. A face-to-face vendor meeting is planned to take place at BfArM in Bonn, Germany on 6th of October 2017. All vendors are invited, at their own cost to take part. All participants must register in advance, by 22nd September 2017, via email to eCTD4consultation@ema.europa.eu.
The eSubmission Gateway Web Client has been updated in the XCOMP environment. This update introduces updated user interface and improved functionality – users no longer need to manually select the large file applet when sending test submissions using the vgateway in XCOMP environment. Please see the mini user guide for the updated test system here. This new update will be made available in the production environment on 23rd of September 2017 if no issues are detected in testing. The user credentials remain the same in the updated system.
10-08-2017
Essential maintenance of affecting eSubmissions
The EudraVigilance XCOMP Gateway will be unavailable between 09:00 and 17:00 on Sunday, 13 August due to essential maintenance. As part of the exercise, the EMA Production Gateway will be unavailable for 30 minutes at a point between 09:00 and 13:00. In consequence, no Production inbound messages relating to eSubmissions or EudraVigilance will be processed during those 30 minutes and no outbound MDN or ACK messages will be sent. There may also be a very brief interruption to other services as the EMA Production Gateway is brought back online.
12-07-2017
A defect in section 1.4 of the MAA veterinary eAF, affecting the MRL substances has been detected and subsequently fixed. New version v.1.21.0.1 of the MAA veterinary form and the updated release notes are now available.
Defects in section 1 and 3 of the Renewal eAF, affecting the MA Numbers fields and the overages field respectively, have been detected and subsequently fixed. New version v.1.21.0.1 of the Renewal form and the updated release notes are now available.
Please note that the Data Exchange Standard (DES) has not changed in this release – the change in the release number reflects that there is no change in the DES. The new version of the forms should be used as of today. Please note that there is no change to the Variation form – the version number 1.21.0.0 will remain for the Variation form.
04-07-2017
PAM Submission Form is now available. The new form aims to provide a better ‘end to end process’ for PAMs by improving the submission and start phase with greater automation, and by increasing transparency for the Applicant with regards to EMA resources allocated to the procedure, evaluation timetables, and scientific committee assigned.
The full functionality of the PAM Submission Form will be achieved when changes to the eSubmission Gateway are implemented on the 1st of September. From this moment on the new form will be mandatory for all PAM submissions.
Further information will be provided via the post-authorisation procedural Q&A, the eSubmissions webpage and a dedicated regulatory news item closer to the go-live date.
Related information: Post-authorisation measures: questions and answers
30-06-2017
A defect in section 1.1 of the MAA human eAF, affecting the PRAC rapporteur field (Centralised Procedure applications) has been detected and subsequently fixed. New version v.1.20.0.1 of the MAA human form and the updated release notes are now available here.
Please note that the Data Exchange Standard (DES) has not changed in this release – the change in the release number reflects that there is no change in the DES. The new version of the forms should be used as of today. The issue in version 1.21.0.0 does not affect MRP/DCP/NP applications. Please note that there is no change to Variation, Renewal and MAA vet forms – the version number v.1.21.0.1 will remain for variation, renewal and vet MAA forms.
Updated release notes are now available for eSubmission Gateway XML delivery file user interface. The updated document highlights a known issue with submission description ‘Response to RSI’ when submission units ‘initial, validation-response, additional-info, closing, consolidating and corrigendum’ are selected. This defect does not affect the submissions process and will be fixed in the next release.
28-06-2017
An updated version of the eSubmission Gateway XML delivery file user interface is now available. The eSubmission Gateway update will introduce improvements, new business rules and new fields to the user interface. This change is a further step towards integration of the XML delivery file and the Formatted Table Template; however this release does not affect the use of the Formatted Table Template. Release notes detailing the changes are available here.
Users should note that the delivery files created using the eSubmission Gateway XML delivery file UI prior to the new release on 27th June 2017 will not work following the deployment of the new version. It is recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues.
27-06-2017
Essential maintenance to computer applications on Tuesday afternoon
PSUR/eSubmissions Web User Interface (to create the delivery file) will be unavailable between 15:00 and 17:00 on Tuesday, 27 June 2017. Users should note that the delivery files created using the eSubmission Gateway XML delivery file UI prior to the new release on 27th June 2017 will not work following the deployment of the new version.
21-06-2017
A presentation on the outcome of the first ‘proof of concept’ UAT on CESSP is now available here.
20-06-2017
Essential maintenance to EMA computer applications affecting PSUR Repository on Saturday, 24 June
PSUR/eSubmissions Web User Interface (to create the delivery file) will be intermittently unavailable between 09:00 and 19:00 on Saturday, 24 June due to essential maintenance. EMA’s gateway will remain available for all communities throughout and any files submitted during that time will be put in a queue for processing once the deployments have been completed.
If you have any questions, please contact the EMA Service Desk.
And
New versions of the 4 electronic Application Forms (eAF v. 1.21) and the related release notes are now available here. This version of the forms provides usability improvements and defect fixes and aims to improve the user experience for all users. This new version, 1.21 of the forms can be used as of today (20th June 2017) and will fully replace the version 1.20.0.5 after transitional period, on1 September 2017. Version 1.20.0.5 of the forms will be withdrawn from the eAF website on 20th June 2017 however it may be used until 31st August 2017. The version of the form should not be changed during an ongoing procedure.
15-06-2017
Version 1.21 of the eAFs will be available on 20 June 2017. The release v1.21 provides usability improvements and technical defect fixes. Following a successful User Acceptance Testing (UAT) the release schedule has now available to provide more information regarding the mandatory use of version 1.21.
An updated version of the eSubmission Gateway XML delivery file user interface and the PSUR Repository is planned for Tuesday 27th June 2017. The eSubmission Gateway UI update will introduce improvements to the user interface and new fields. This change is a further step towards integration of the XML delivery file and the Formatted Table Template; however this release does not affect the use of the Formatted Table Template.
Users should note that in some cases delivery files created prior to the new release on 27th June 2017 may not work following the deployment of the new versions. It is recommended to clear the cookies and refresh your browser before creating delivery files using the updated user interface to avoid any issues.
An interactive training session introducing the changes on the eSubmission Gateway delivery file will be held on Monday 26th June at 11am UK time. Attendance is on first come first serve basis.
To register to join the training please contact: eSubProgOfficer@ema.europa.eu by Friday 23rd June 2017.
06-06-2017
Essential IT maintenance to EudraVigilance applications the Weekend of June 17th 2017
Due to essential maintenance between Friday, 16th June 2017 17:00hrs and Sunday 18th June 2017 23:30hrs (UK time), EudraVigilance Human (for ICSR and Product) and EudraVigilance Veterinary (including EV Web and Gateway) will be unavailable. For any questions, please contact the EMA Service Desk.
20-05-2017
An updated version of the eCTD v4.0 EU M1 Implementation Guide is now available for public consultation. The eSubmission Change Management Board invites all pharmaceutical companies, vendors of eCTD building and reviewing tools and competent authorities to take part in this consultation and provide comments by 30th September 2017. Please provide your comments using the comment template; and any questions regarding further development and clarifications of the released draft documents to: eCTD4consultation@ema.europa.eu. The Implementation Guide and relevant documents can be found here.
The Annex VI of the eSubmission Roadmap on the implementation of mandatory use of VNeeS format for Veterinary regulatory submissions has been updated to reflect the updated eSubmission Roadmap v2.0 and is now available here.
09-05-2017
Version 1.21 of the eAFs will become available on 20 June 2017. The release v1.21 will provide usability improvements and technical defect fixes.
We are organising a User Acceptance Testing (UAT) to support the release of this next version of the forms:
- The testing by Industry and NCAs will take place
- Industry: from Mon 22/05/17 to Fri 26/05/17
- NCAs*: from Mon 29/05/17 to Fri 02/06/17 (*Based on the eAFs received from Industry)
If you wish to participate, please email eSubprogofficer@ema.europa.eu. A feedback form for consolidated comments will be provided to you following your registration.
5-05-2017
An updated version of the eSubmission Gateway Web UI will be made available today at 4pm Friday 5th of May. This new version delivers defect fixes and addresses a data protection concern as the application contact person name and contact person contact details are no longer visible in the user interface and the XML delivery file. Details on the defect new fields are described in the updated release notes and the updated User Guide which are available here.
An editorial change has been made to eCTD EU Module 1 specification. The updated EU M1 spec v3.0.2 is available here. There are no changes to the DTD or the validation criteria; and hence this version enters into force as of today and should be used immediately. The users are recommended to use the new version as soon as possible.
04-05-2017
Essential maintenance to computer applications on Friday afternoon
PSUR/eSubmissions Web User Interface (to create the delivery file) will be unavailable between 15.00 and 16.00hrs on Friday, 5 May 2017. However, the Axway gateway will remain available for all communities and any files affected by the work submitted during that time will be put in a queue for processing when the deployments have been completed. If you have any questions, please contact the IT Service Desk.
19-04-2017
Essential IT maintenance to EudraVigilance applications the Weekend of April 30th
Due to essential maintenance between Friday, 28th April 2017 17:00hrs and Sunday 30th April 2017 23:30hrs (UK time), EudraVigilance Human (for ICSR and Product) and EudraVigilance Veterinary (including EV Web and Gateway) will be unavailable. For any questions, please contact the EMA Service Desk.
06-04-2017
Presentation from the training session held on 3rd April 2017 on the use of the updated eSubmission Gateway XML delivery file user interface; and the updated Annex I on the use of the filenaming conventions for eSubmission Gateway are now available here.
A presentation on the Common European Single Submission Portal (CESSP) is available here. The presentation explains the essentials on CESSP with background and details of the project.
eCTD EU Module 1 specification has been updated. The updated EU M1 spec v3.0.2 and the related release notes are available here. There are no changes to the DTD and hence this version enters into force as of today and can be used immediately. The users are recommended to use the new version as soon as possible.
Question and Answer document on the eCTD validation criteria is now available here.
31-03-2017
An updated version of the eSubmission Gateway Web UI is now available. This version delivers new functionality to provide information currently provided in the Formatted Table Template. The aim of this phased implementation is to stop using the template once all relevant fields are available in the XML delivery file. This particular release does not affect the use of the Formatted Table Template. Details on the new fields are described in the updated release notes and the updated User Guide available here. The users should note that in some cases delivery files created prior to the new release on 31st March 2017 for referral and CAP submissions may not work following the deployment of the new versions.
An updated version of the PSUR Repository is now available. This minor release provides defect fixes and corrected business rules. Updated release notes are available here.
The use of the eCTD Specification v3.0.1 is mandatory from 1st April 2017. The EU Module 1 Specification is available here.
The use of XML delivery files for all Veterinary submissions is mandatory from 1st April 2017. See the Veterinary eSubmission page for more information.
And
POSTPONED: Essential IT maintenance to EudraVigilance applications the Weekend of April 1st 2017
The scheduled IT maintenance between Saturday 1st April 2017 06:00hrs and Sunday 2nd April 2017 23:30hrs (UK time) to EudraVigilance Human (for ICSR and Product) and EudraVigilance Veterinary (including EV Web and Gateway) has been postponed and the applications will remain available throughout this period. Revised dates will be announced in due course.
29-03-2017
Essential maintenance to computer applications on Friday morning
PSUR/eSubmissions Web User Interface (to create the delivery file) will be unavailable between 07.00 and 09.00hrs on Friday, 31 March 2017. However, Axway gateway will be available for all communities and any files submitted during that time will be put in a queue for processing when the deployments will be completed.
28-03-2017
An updated version of the eSubmission Gateway XML delivery file user interface and the PSUR Repository will be available in the morning on Friday 31st of March 2017. The eSubmission Gateway UI update will introduce new fields for applicants/MAHs to provide submission description and other additional details currently provided on the Formatted Table Template. This change is a first step towards adding this information in the delivery file; however this release does not affect the use of the Formatted Table Template.
Users should note that in some cases delivery files created prior to the new release on 31st March 2017 for referral and CAP submissions may not work following the deployment of the new versions.
An interactive training session introducing the changes on the eSubmission Gateway delivery file will be held on Monday 3rd April at 11am UK time. Attendance is on first come first serve basis. Please join the meeting in the virtual meeting room: https://connect.ema.europa.eu/webinar/
24-03-2017
The Annex 2 of the eSubmission Roadmap on the implementation of mandatory use of eCTD format for regulatory submissions has been updated to reflect the updated eSubmission Roadmap v2.0 and is now available here.
16-03-2017
Essential IT maintenance to EudraVigilance applications the Weekend of April 1st 2017
Due to essential maintenance between Saturday 1st April 2017 06:00hrs and Sunday 2nd April 2017 23:30hrs (UK time), EudraVigilance Human (for ICSR and Product) and EudraVigilance Veterinary (including EV Web and Gateway) will be unavailable. For any questions, please contact the EMA Service Desk.
08-03-2017
An updated version of the eSubmission Roadmap has been published. The Roadmap has been updated to reflect achieved milestones and to include new and amended timelines for implementation of various telematics systems/standards. The Annexes related to the eSubmission Roadmap will be updated in line of the changes detailed in the roadmap. The updated annexes will be published on the CMB website once available. The updated Roadmap v2.0 and the visual representation of the steps are available here.
23-02-2017
A defect in section 2 of variation eAF causing fields to overlap and an issue affecting the co-rapporteur field in the human MAA form have been detected and subsequently fixed. New version v.1.20.0.5 of the variation and MAA human forms and the updated release notes are now available here. Please note that the Data Exchange Standard (DES) has not changed in this release – the change in the release number reflects that there is no change in the DES. The new versions of the forms can be used as of today and the version 1.20.0.5 will fully replace previous versions on1 March 2017. Please note that there is no change to Renewal and MAA vet forms – the version number 1.20.0.4 will remain for renewal and vet MAA forms.
20-02-2017
An updated version of the eSubmission Gateway XML delivery file web UI is now available. This version delivers a change to the Referral submissions by including information from the Referral cover letter in to the XML delivery file and hence simplifying the submission process and allowing further automation. Defect fixes have also been implemented. The User guide to the XML delivery file creation and the Release notes have been updated and are now available here.
An updated version of the PSUR Repository is now available. This release provides new functionality, changes to the PSUR Repository notifications and defect fixes. The updated NCA User Guide and updated MAH User Guide as well as the updated release notes are available here.
16-02-2017
Essential maintenance to PSUR Repository
PSUR/eSubmissions Web User Interface (to create the delivery file) will be unavailable between 07.00 and 09.00hrs on Monday, 20 February 2017. However, the Axway gateway will be available for all communities and any files submitted during that time will be put in a queue for processing when the deployments will be completed.
07-02-2017
Updated versions of 4 electronic Application Forms (eAF v. 1.20.0.4 – initially called v1.21) are now available. This new release provides usability improvements and defect fixes with an aim to further improve user experience. Please note that the Data Exchange Standard (DES) has not changed in this release – the change in the release number reflects that there is no change in the DES. The updated forms and the related release notes are now available here. The new versions of the form can be used as of today and the version 1.20.0.4 will fully replace version eAF v.1.20.0.3 after a transitional period on1 March 2017. The version of the forms should not be changed during an ongoing procedure.
11-01-2017
Version 1.21 of the eAFs will become available on 7 February 2017. The release v1.21 will provide mainly technical defect fixes and usability improvements.
We are organising a User Acceptance Testing (UAT) to support the release of this next version of the forms:
- The testing by Industry and NCAs will take place
- Industry: from Mon 16/01/17 to Thu 19/01/17
- NCAs*: from Tue 23/01/17 to Fri 27/01/17 (*Based on the eAFs received from Industry)
If you wish to participate, please email Beatriz.Calvo@ema.europa.eu. A feedback form for consolidated comments will be provided to you following your registration.
Please use the Service Desk portal https://servicedesk.ema.europa.eu to raise any queries related to the eAFs. Procedural queries related to MRP, DCP and National procedures should be directed to the relevant National Competent Authority.
13-12-2016
New versions of the VNeeS Guideline v2.5 and the related Validation checklist v2.5 have been published under the Current Guidance section on the Vet eSubmission website. The new VNeeS Guideline and the checklist will enter into force on 1 June 2017. Rules for technical validation remain unchanged and therefore the current VNeeS Checker v.2.4b (validation tool) will remain valid from 1 June 2017.
The release schedule for the update of the electronic Application Forms (eAFs) is now available and can be found here. The next release, version 1.21 will deliver defect fixes and usability improvements and is planned for user acceptance testing in January 2017 and for deployment in February 2017.
The User guide to the XML delivery file creation has been updated and is available here.
Support to eSubmissions during EMA holidays: Please note that all EMA eSubmission systems are running as per normal during the EMA holiday. Submissions received via the eSubmission Gateway during the EMA closure are automatically uploaded to the Common Repository and the PSUR Repository as relevant. In case of technical issues, the EMA IT service desk is open until 6:30pm UK time on 22nd of December 2016. The service desk will reopen for external users for 4 half days between 27th and 30th December 2016, please note that if the queries are complex or require specific expertise they may not be solved until the service desk returns to normal service on Tuesday 3rd January 2017. There will be no procedural support available during the EMA closure from 23rd December until 2nd of January 2017 inclusive.
05-12-2016
An updated version of the eSubmission Gateway XML delivery file web UI is now available. This version delivers new functionality to create delivery files for MRL and veterinary PSUR submissions as well as functionality to create delivery files for human PASS 107o submissions, PASS 107n, PASS 107o and PASS 107q submissions for Nationally Authorised Products and submissions for Ancillary Medicinal Products in medical devices.
The User guide to the XML delivery file creation and the Release notes have been updated and are now available here.
The use of eSubmission Gateway / Web Client will become mandatory for all Veterinary submissions on 1 January 2017. The procedural announcement and presentation from the training held on 1 December 2016 are available here.
The use of XML delivery files for all Veterinary submissions will become mandatory on 1 April 2017. The Statement of Intent is available here.
The Common Repository will be extended to all veterinary submissions in the centralised procedure. The Statement of Intent is available here.
The use of VNeeS format for all veterinary submissions in European procedures (CP, DCP and MRP) will become mandatory as of 1 January 2017. Details are available in the Annex to the eSubmission roadmap.
01-12-2016
Essential maintenance to computer systems during the weekend
Please note that the following computer systems will be unavailable on Sunday, 4th December 2016 between 9.00hrs and 19.00hrs (UK Time) due to essential maintenance:
- PSUR and eSubmission Web User Interface – affecting parts of DREAM*
- Axway Gateway – affecting EudraVigilance and eSubmissions
(* Due to essential folder re-structuring, colleagues working in the cabinet 01 /H-PUSA area might receive warning messages. Please avoid working in this area on Sunday 4th Dec, if possible. All other areas of DREAM will be available as usual.)
22-11-2016
A statement of intent is published regarding the replacement of electronic Application Forms by CESSP. More information on the CESSP project is available here.
11-11-2016
As of 1 January 2017 it will be mandatory for applicants to make all veterinary procedural submission to EMA via the eSubmission Gateway/Web Client portal. In preparation, a training Webinar for Industry – Mandatory Use of eSub Gateway for veterinary submissions to EMA will be held on 1 December 2016 from 13:00 to 15:00 UK time. For details see the Veterinary esubmission website.
09-11-2016
An updated version of the Common Repository is now available for the NCAs. The Common Repository contains all Centralised Procedure eCTD format submissions and is accessible to all NCAs. The update user guide is now available for NCAs. The updated Q&A document is now available here.
And
The Practical User Guide for Electronic Application Forms (eAF) for human and veterinary medicinal products in the EU has been updated and is available here.
07-11-2016
Essential maintenance to replace the Common Repository web user interface
The European Medicines Agency will be carrying out essential maintenance activities on the Common Repository system during the period specified below. Users may therefore find the application being intermittently unavailable during this time.
Tuesday, 8 November 2016 18:00hrs - 20:00hrs (UK time)
This maintenance activity will introduce a new web application to users which will replace the existing Common Repository web user interface. The new version of the system provides access to the word version working documents via the user interface and introduces a new look and feel with EMA branding. An updated user guide has been circulated to Common Repository contact points and is also available on the Telematics website.
We apologise for any inconvenience caused. If you have any questions, please contact the IT Service Desk.
18-10-2016
Updated versions of 4 electronic Application Forms (eAF v. 1.20.0.3) have been published following reported issues. The updated forms and related release notes are now available here.
The User guide to the XML delivery file creation has been updated and is now available here.
14-10-2016
The Annex1 - A Guide to the EMA filenaming convention for eSubmission has been updated and is available here.
Essential maintenance to PSUR Repository and eSubmission Gateway this weekend
Due to essential maintenance the PSUR Repository and the eSubmission Gateway are unavailable this weekend from 18:00 UK time on Friday, 14 October until 06:00 AM UK time on Monday 17 October 2016.
EMA IT systems unavailable from 18:00 Friday 28 October to 06:00 Tuesday 1 November
All European Medicines Agency (EMA) IT systems will be temporarily unavailable from 18:00 on Friday 28 October to 6:00 on Tuesday 1 November (UK time), due to an essential exercise to test the Agency’s IT recovery capacity in case of a major event.
During this period, it will not be possible to access the EMA public website, www.ema.europa.eu, or any other EMA-hosted website or online application, including the systems used to send and receive applications for EMA regulatory procedures. MAHs should plan their submissions around the availability of the systems.
Emails sent to EMA email addresses during this period will be queued and delivered to recipients on 1 November.
Normal service will resume on 1 November.
The EMA product emergency hotline and phone number for notifying suspected quality defects or product recalls will operate as usual. While the EMA public website is unavailable, a holding page will be displayed with details of these emergency numbers. We would like to thank you in advance for your patience and cooperation during this essential IT maintenance work.
If you require more information, please contact the IT Service Desk ITServiceDesk
10-10-2016
An updated version of the PSUR Repository has been made available on Thursday 6th October 2016. This release improves the product availability for the submission of responses. The updated release notes are available here.
An updated version of the eSubmission Gateway XML delivery file web UI is now available. This version improves the availability of Referral and ASMF procedures as well as fixing known defects. The updated release notes are available here.
04-10-2016
Essential maintenance to PSUR Repository on Thursday evening.
PSUR/eSubmissions Web User Interface (to create the delivery file) will be unavailable between 18:00 and 19:00hrs on Thursday, 6 October 2016. However, Axway gateway will be available for all communities and any files submitted during that time will be put in a queue for processing when the deployments will be completed.
If you have any questions, please contact the EMA IT Service Desk
03-10-2016
Essential maintenance to Gateway - 14-17 October 2016
The European Medicines Agency will be carrying out essential maintenance activities on the Gateway during the period specified below. Users may therefore find the Gateway unavailable during this time.
- Friday, 14th October from 20.00hrs (UK time) to Monday, 17th October, 6.00hrs (UK time)
During this period, it will not be possible to send messages via the Agency’s Gateway. Depending on the routing, either there will be a failure to connect and the message will not reach the Agency, or the message will be queued and delivered once services have been restored. Only in the second case will an MDN be received and the message processed once the service has been restored. Users should therefore plan their submissions around the availability of the systems.
Normal service will resume at 6.00hrs (UK time) on Monday, 17 October 2016.
We would like to thank you in advance for your patience and co-operation during this essential IT maintenance work and we apologise for any inconvenience this may cause. Should you have any questions please contact the EMA IT service desk at https://servicedesk.ema.europa.eu
Please Note:
We have added an RSS feed on the Home page which allows you to get any updates to the site instantly. You are encouraged to subscribe to this feed. To subscribe click on the above RSS feed icon and follow the instructions.
01-10-2016
The use of the updated EU Module 1 Specification v3.0 and/or v3.0.1 is now mandatory for all eCTD format submissions in the EU. The specification and related documents are available here.
The use of the XML delivery files for submissions via the eSubmission Gateway and the Web Client is now mandatory. The use of XML delivery files has replaced the use of filenaming conventions for majority of human and veterinary submissions to the EMA. More information on the use of the delivery files for submissions via the eSubmission Gateway and the Web Client is available here. The Annexes describing the filenaming conventions have been updated.
Applicants are reminded that the section 2 of the electronic Application Form (eAF) should be filled in. The use of the footnote is only allowed for form and strength information for complex vaccines/combination products. The eAFs and related user guides are available here.
Applicants are reminded that eCTD validation reports must not be provided in the working documents folder in the submissions to EMA. More information on the use of the working documents folder is available in the Harmonised Technical Guidance for eCTD Submissions in the EU document.
19-09-2016
A further update to the revised Annex II to the HMA eSubmission Roadmap on the implementation of mandatory use of eCTD format for regulatory submissions is available here.
The use of the eAF v1.20 will become mandatory on Monday 19th September 2016. Use of previous versions is accepted only for ongoing applications – the version of the form should not be changed in the middle of the procedure. The latest version of the forms and relevant guidance is available here.
Essential maintenance to PSUR Repository and the eSubmission Gateway on Tuesday evening 20th September 2016
Please note that the PSUR Repository will be unavailable for Member State and industry users between 18.00-19.00hrs UK time on Tuesday, 20th September due to essential maintenance. The EMA eSubmission Gateway/Web Client will not be available during this time.
If you have any questions or issues, please contact the EMA IT Service Desk
19-08-2016
Updated versions of 4 electronic Application Forms (eAF v. 1.20.0.2) have been published following reported issues. The updated forms and related release notes are now available here. Version 1.20 of the forms has been available for use since 14th June 2016 and will fully replace version eAF v. 01.19 after an extended transitional period on19 August 2016. Version 1.19 of the forms may be used until 18 September 2016.
18-08-2016
Recordings from the webinar training on the use of the updated XML delivery files for submissions via the eSubmission Gateway held on 21st July and the interactive Q&A sessions held on 25 and 26th July are now available here.
The recording of the PSUR Repository v1.07 webinar training held on 25 July and recordings from the webinars on the Mandatory use are now available here
27-07-2016
Common Repository unavailable on Thursday evening 28 July 2016 for scheduled maintenance.
The Common Repository will be unavailable on Thursday 28 July 2016 from 18.00 until 21.00 UK time while scheduled upgrade work is carried out. This upgrade introduces changes to bring the Common Repository into line with eCTD EU Module 1 specification v3.0.
And
The updated release schedule for the hotfix version 1.20.0.2 for electronic Application Forms has been published here. The acceptability of the v1.19.0.2 of the eAF has been extended until 19 September 2016 when the updated versions of all 4 forms plan to be published.
The updated eSubmission Gateway error codes list is now available here. The error codes listing provides problem descriptions for errors experienced by users when sending packages using the eSubmission Gateway / Web Client.
The updated formatted table template is now available here. The main changes in this cover letter template reflect changes in the updated eCTD EU Module 1, the usage of the template for non-EU single assessment submissions and the discontinued use of Annex I for product listing for PSUSA procedures (product listing is created when products are selected on the xml delivery file).
Updated Annex II to the HMA eSubmission Roadmap on the implementation of mandatory use of eCTD format for regulatory submissions is available here.
The presentation from the training session on the use of the updated version of the PSUR Repository held on 25 July 2016 is available here.
The presentation from the interactive Q&A sessions held on 25 and 26 July 2016 on the use of the updated version of eSubmission Gateway XML delivery file user interface is now available here.
25-07-2016
Updated versions of the PSUR Repository and the eSubmission Gateway user interfaces to create XML delivery files for submissions via the eSubmission Gateway and the Web Client are now available. These new versions will introduce changes in line with the updated EU Module 1 Specification v3.0 and v3.0.1. The updated delivery files should also be used for eCTD submissions created using eCTD specification version 2.0. The changes are implemented to the Gateway XML delivery files also for Veterinary submissions to align the submission process and provide more information on the submission.
Updated user guides and system release notes are available for PSUR Repository users here and for Gateway users here. Slides from the training session held on 21 July 2016 on the use of the updated delivery files are now available here.
Issues have been reported with the latest versions of the electronic Application Forms v1.20.0.1 and, while the issues are fixed the acceptability of the v1.19.0.0 of the eAF has been extended until 15 September 2016. The new versions of all the 4 forms are planned to be published within the coming weeks, exact date of this new release is yet to be confirmed.
If you are experiencing issues with the forms, please contact the EMA IT service desk via the online portal: https://servicedesk.ema.europa.eu.
The formatted table template cover letter has been updated and is now available here.
22-07-2016
Please Note Monday, 25 July 2016 07:00hrs – 09:00hrs (UK time):
The European Medicines Agency will be carrying out essential maintenance activities on the PSUR/eSubmissions Web/Gateway File Handler during the period specified below. Users may therefore find the application being unavailable during this time. However, Axway gateway will be available for all communities and any files submitted during that time will be put in a queue for processing when the deployments will be completed.
14-07-2016
Updated versions of the PSUR Repository and the eSubmission Gateway user interfaces to create XML delivery files for submissions via the eSubmission Gateway and the Web Client will become available by 1st of August 2016. These new versions will introduce changes in line with the updated EU Module 1 Specification v3.0. The changes are implemented to the Gateway XML delivery files also for Veterinary submissions to align the submission process and provide more information on the submission.
Invitations to training sessions and interactive Q&A sessions have been sent to registered Gateway users and contact points who have previously attended PSUR Repository training. If you have not received an invitation and would like attend, please register by contacting eSubProgOfficer@ema.europa.eu by Wednesday 20th July noon UK time.
The maximum capacity of the virtual meeting rooms is 200 participants and spaces will be allocated on a first come first serve basis. There are 2 separate Q&A sessions to allow as many participants as possible to attend this interactive session. The participants should select one of the sessions only.
If multiple users from your organisation intend to attend, please consider sharing the connection with your colleagues. Sessions will be recorded and the recordings will be made available on the eSubmission website.
| Session |
Date |
Time |
Training on the use of XML delivery files for submissions via the eSubmission Gateway – update reflecting the updated EU M1. |
Thursday 21 July 2016 |
09:00 UK time |
Training and Interactive Q&A on the v1.07 of the PSUR Repository |
Monday 25 July 2016 |
10:00 UK time |
Interactive Q&A session on the use of the XML delivery files for submissions via the eSubmission Gateway (session 1) |
Monday 25 July 2016 |
12:00 UK time |
Interactive Q&A session on the use of the XML delivery files for submissions via the eSubmission Gateway (session 2) |
Tuesday 26 July 2016 |
10:00 UK time |
Updated Release Notes and Implementation Guide have been published to clarify the changes and requirements related to the update of the eCTD EU Module 1 Specification v3.0 and v3.0.1. Both documents can be found from here.
Updated Release Notes have been published to provide clarification on the changes and the use of the eCTD Validation Criteria v6.1. The updated document can be found here.
Issues have been reported with the latest versions of the electronic Application Forms v1.20.0.1 and, while the issues are being investigated and fixed, new versions of all the 4 forms are planned to be published within the next coming weeks, exact date of this new release is yet to be confirmed. If you are experiencing issues with the forms, please contact the EMA IT service desk via the online portal: https://servicedesk.ema.europa.eu.
04-07-2016
ASMF: It is now mandatory to use eCTD format for all ASMF submissions for human use in the centralised procedure.
eCTD: The EUM1 Specification v3.0 and v3.0.1 have entered into force on 1st July 2016 and may be used for all eCTD format submissions within Europe.Submissions in v2.0 will be accepted until 30th September 2016.
VNeeS: An updated version of the VNeeS checker version 2.4.b has been published on the websites of the French and Belgian competent authorities. More information on the update and information on the veterinary eSubmissions can be found here.
eSubmission Gateway: Presentation and recording from the webinar training on the use of XML delivery files for Veterinary submissions via the eSubmission Gateway, held on 3 June 2016, are now available here.
The recordings from the training session and the interactive Q&A session on the use of XML delivery files for submissions via the eSubmission Gateway are now available here.
30-06-2016
eAF: Updated versions of all 4 electronic Application Forms (eAF v. 1.20.0.1) have been published following reported issues with the proof of payment section and entering quantities in the initial human MAA form. The updated forms and related release notes are now available here. Version 1.20 of the forms may be used from 14th June 2016 and will fully replace the current version of eAF v. 01.19 after a transitional period on1 August 2016. Version 1.19 of the forms may be used until 1 August 2016.
PSUR Repository: In some cases, when submitting a package to the PSUR Repository, the zip manager did not create a specific entry for the top level sequence directory. This caused the system to generate an error since it could not match the sequence number provided in the xml delivery file with the top level directory of the zip file.
This issue has now been resolved and, in all cases, the zip manager creates a specific entry for the top level sequence directory. More information on the mandatory use of the PSUR Repository can be found here.
24-06-2016
Variation eAF: Due to an unexpected technical issue the publication of the updated version of the form is postponed to early next week. The hotfix version will be made available as soon as possible during the week commencing 27th June 2016.
ASMF: Updated draft version of the Practical Guidance on the use of the eCTD format for ASMFs for Active Substance Master File Holders and Marketing Authorisation Holders has been released for public consultation and is available here. Please send your comments to EUM1Spec@ema.europa.eu by 29 July 2016.
21-06-2016
Variation eAF: Two issues have been detected with the new version of the variation application form (eAF v. 1.20) affecting the use of the form in its current state for MRP/DCP applications. EMA is currently fixing these defects affecting selection of Reference Member State (MRP/DCP procedures) and the Proof of Payment section. The form may continue to be used for Centralised Procedure applications and National Procedure applications. A new version of the form will be published later this week. If you have already started to fill in the application form v. 1.20 and you are submitting an application prior to the publication of the hotfix version, please provide the information from Proof of Payment section as a separate annex to the application form. Alternatively, you may use version 1.19. Please note that once the hotfix version becomes available the information already filled in v. 1.20 can be imported to the new version. We would like to thank those users who have reported the issues with the forms.
Important Notice to all PSUR Repository users and MAHs:
The PSUR Repository will be temporarily unavailable from 09:00 – 12:00 (UK time) on Saturday 25 June 2016 due to essential maintenance. All submissions sent to the PSUR repository during the downtime are queued and will be made available in the system once normal service is returned.
14-06-2016
New versions of the electronic Application Forms (eAF v. 1.20) and the related release notes are now available here. This version of the forms provides usability improvements and defect fixes and aims to improve the user experience for all users. Version 1.20 of the forms may be used from 14th June 2016 and will fully replace the current version of eAF v. 01.19 after a transitional period on1 August 2016. Version 1.19 of the forms will be withdrawn from the eAF website on 1 July 2016 however it may be used until 1 August 2016. The use of the eAFs is mandatory for all human and veterinary submissions.
10-06-2016
The use of the PSUR Repository is mandatoryas of 13 June 2016. All PSURs for products authorised in Europe must now be submitted to the PSUR Repository (PSURs for products authorised under Art. 58 are excluded). There should be no PSUR submissions directly to the National Competent Authorities from Monday 13th June 2016 onwards. More information on the mandatory use of the PSUR Repository as well as the updated user guidance is now available here.
07-06-2016
The Data Exchange Standard (DES) for the variation eAF has been updated reflecting changes implemented in the upcoming release of all electronic Application Forms (eAFs) version 1.20 on 14th June 2016. The variation DES and the DES change summary are now available here.
06-06-2016
The interactive Q&A session on the use of the xml delivery files for submissions via eSubmission Gateway scheduled for 6th of June at 10am has been cancelled due to technical issues with Adobe connect tool. A new date will be organised and all registered users will be invited to the session.
01-06-2016
- An error has been detected in the published md5 checksum and the update is now available here.
- Updated release notes for the eCTD validation criteria v6.0 and v6.1 are now available here.
- Updated release notes for the eCTD EU M1 specification v3.0.1 are now available here.
- All questions, queries and issues on the use of the PSUR Repository should now be reported via the EMA service portal: https://servicedesk.ema.europa.eu. Please indicate in your query that the issue concerns PSUR repository. The introduction of the EMA service portal aims to harmonise the process to report and track queries sent to EMA. The PSURrepository@ema.europa.eu email address should be to submit change requests related to PSUR repository system.
- Interactive Questions and Answers sessions are being organised on the use of the XML delivery files for all submissions via eSubmission Gateway/Web Client. Please note that both sessions; 1.6.2016 at 12:00 – 13:30 UK time and 6.6.2016 at 10:00-11:30 UK time are on a first come first serve basis, and only the first 200 participants are able to connect to the sessions.
Wherever possible, please share the connection with your colleagues and if you are unable to attend the recordings for both of these Q&A sessions will be published on the eSubmission Gateway website.
Further training and interactive Q&A sessions are planned for July 2016 once a new version of the system is released to reflect the updated EU Module 1 specification.
- Presentation from the training on the use of the XML delivery files for eSubmission Gateway / Web Client is now available here.
- The user guide on XML delivery file creation has been updated and is available here.
23-05-2016
An eSubmission Gateway user interface to create XML delivery files for submissions via the eSubmission Gateway and the Web Client is now available. The use of the delivery files is initially introduced as a pilot, covering the majority of submission types for both human and veterinary domains. Use of the delivery files will become mandatory for Human Centralised Procedure submissions in line with the mandatory use of the updated eCTD EU M1 in October 2016. The use of filenaming conventions will be phased out after a transitional period for all other procedure types. The user guide to XML delivery files and the release notes are available here.
A webinar on the Introduction to new Gateway User Interface for Veterinary Applicants will be held on 3 June 2016 from 11.30 to 13:00.00 UK time. This webinar is dedicated to Veterinary Industry. Please contact eAFwebinar-registration@ema.europa.eu by 1 June 2016 to register and to send any queries you would like to addressed during this webinar.
19-05-2016
A new release of eSubmission Gateway user interface will introduce use of XML delivery files for submissions via the eSubmission Gateway and the Web Client, replacing currently used filenaming conventions. The use of the delivery files is initially introduced as a pilot, covering the majority of submission types for both human and veterinary domains.
A webinar on the use of the new Gateway User Interface for Applicants for Human medicinal products and PIP sponsors will be held on 23 May 2016 from 10:00 to 11:30 UK time. A meeting invitation to the session with connection details will be sent to all registered Gateway users.
A webinar on the Introduction to new Gateway User Interface for Veterinary Applicants will be held on 3 June 2016 from 11.30 to 13:00.00 UK time. This webinar is dedicated to Veterinary Industry. Please contact eAFwebinar-registration@ema.europa.eu by 1 June 2016 to register and to send any queries you would like to be addressed during this webinar.
Should several participants from the same company wish to attend, it is advised to send only one registration request and attend from the same room. Both sessions will be recorded and the recording will be made available on the eSubmission website.
17-05-2016
Due to am error in the EU M1 specification v3.0, a new version (3.0.1) with the updated md5 checksums is now available here.
13-05-2016
Version 01.06 of the PSUR Repository will be made available for both MAHs and NCAs on 14th of May 2016. This release provides improvements to the system prior to the start of the mandatory use on 13th June 2016. The release also provides fixes for defects that have been identified during simulated mandatory use. While the new version is being made available the system will not be able to process new submissions. The PSUR Repository will be unavailable for MAH and NCA users between 9:00 am and 9:00 pm UK time on Saturday 14th May 2016. The updated system release notes and MAH and NCA user guides can be found here.
12-05-2016
A series of trainings and Q&A sessions have been scheduled in preparation for the upcoming PSUR Repository release v1.06.00 and for the mandatory use.
The industry representatives are encouraged to register for the training by sending contact details to eSubprogofficer@ema.europa.eu. NCA representatives will receive an invitation from EMA without a need to register.
11-05-2016
The PSUR Repository bulletin has been updated and can be found here.
10-05-2016
Version 01.06 of the PSUR Repository will be made available for both MAHs and NCAs on 14th of May 2016. The new release provides change requests and defect fixes further enhancing the system in preparation for the mandatory use starting on 13th June 2016. While the new version is made available the system will not be able to process new submissions. Submissions will be received via the eSubmission Gateway however will only be made available in the PSUR repository once the deployment is finalised. The PSUR Repository will be unavailable for both, MAH and NCA users between 9:00 am and 9:00 pm UK time on Saturday 14th May 2016. More information on the PSUR Repository can be found here.
03-05-2016
The EU Harmonised technical eCTD guidance has been updated. The updated document is available here.
Due to the high volume of feedback received for the eAF UAT, the release date has been revised. The updated release schedule is available here.
Annex 1 – A guide to the filenaming convention for eSubmission describing the detailed filenames for submission via the eSubmission Gateway / Web Client has been updated and can be found here.
From 23 May 2016 it will be possible to use XML delivery files instead of existing filenaming conventions for submissions via the eSubmission Gateway / Web Client. Results of the user acceptance testing (UAT) in April were successful and more information on the pilot launch of the XML delivery files for all types of procedures will be published this month.
12-04-2016
Publication of eAF version 1.20: Please note that following a very high number of testing feedback for the new version of the eAF, we are revising the publication date and the related release plan to ensure that a user friendly robust form will be released. The new date will be communicated as soon as possible. We apologise for any inconvenience.
A second webinar for NCAs on eAF: Overview and best practices how to automatically import data from eAFs into NCA IT-systems will be held on 15 June 2016, from 10:00 to 11:30 CET. Registrations are accepted until 13 June 2016. For more information and to register, please visit the NTC website.
11-04-2016
Important Notice to MAHs:
All EMA IT systems temporarily unavailable from 06:00 on Saturday 16 April to 06:00 on Sunday 17 April 2016 (UK time) due to essential maintenance. More information is available here.
The Annex 1 – A guide to the filenaming convention for eSubmission document describing the detailed filenaming conventions for submissions via eSubmission Gateway has been updated with new, additional filenaming conventions for ancillary medicinal substances, PASS 107, Signal Detection, NAPs included in a worksharing procedures and Clinical Trial Publication submissions. The updated Annex 1 can be found here.
The PSUR Repository bulletin has been updated and can be found here.
04-04-2016
EU M1 v3.0 Implementation Guide is now finalised and is available here. Minor changes (deleting words ‘county specific’ in 11.9, ordering of 11.BP1/BP2 and 14.1/14.5) have been implemented in the published eCTD validation criteria available here.
29-03-2016
An error had been detected in the eCTD published validation criteria. The updated version with correct md5 checksums is available here.
22-03-2016
From 23 May 2016 it will be possible to use XML delivery files instead of the existing filenaming conventions for number submission types via eSubmission Gateway/Web Client. We would like to involve industry representatives in the upcoming User Acceptance Testing (UAT) which is planned to take place with human and veterinary industry representatives:
- From Monday 18/04/16 to Friday 22/04/16
If you wish to participate in the testing of the new functionality, please email turi.salami@ext.ema.europa.eu. Detailed information package for testing and a feedback form for comments will be provided to you following your registration.
15-03-2016
MAHs are advised that due to system maintenance work the product refresh for the PSUR Repository will be stopped during the following planned dates, which are currently:
- 11th March to 18th March 2016
- 22nd March to 29th March 2016
As a consequence MAHs that make changes to data in Article 57 during these periods will be affected when searching for their products during the XML delivery file creation process.
If you encounter issues with the submission of your initial PSUR we recommend that you wait until these days have passed; there are no planned submission deadlines until 20.04.2016 therefore a slight delay in submission will not impact your procedure start.
If you encounter issues submitting supplementary information please await until the period is over and attempt to resubmit. Please inform your procedure manager (PM) of this and we will communicate with the assessment team accordingly. In those cases where you have an extensive response please highlight this to your PM in order to consider whether an alternative route of submission to the NCA should be considered.
09-03-2016
Version 1.20 of the eAFs will become available on 15 April 2016. To support the release of this next version of the forms:
- New versions of the eAF Data Exchange Standards (DES) and schemas have been released and can be found here.
- We are organising User Acceptance Testing (UAT) of version 1.20 of the eAFs. The testing by Industry and NCAs will take place
- Industry: from Mon 21/03/16 to Thu 24/03/1
- NCAs*: from Tue 29/03/16 to Fri 01/04/16 (*Based on the eAFs received from Industry)
If you wish to participate, please email turi.salami@ext.ema.europa.eu. A feedback form for consolidated comments will be provided to you following your registration.
A webinar for NCAs on eAF: Overview and best practices how to automatically import data from eAFs into NCA IT-systems has been moved from 23 February 2016 to 16 March 2016, from 13:00 to 14:30 CET. This webinar is dedicated to National Competent Authorities and is organised in collaboration with the Network Training Centre. Further registrations are accepted until 14 March 2016. For more information and to register, please visit the NTC website.
The Service Desk portal (https://servicedesk.ema.europa.eu) should be used to raise any queries related to the eAFs.
07-03-2016
The EMA is extending the use of XML delivery files for all submissions excluding the Veterinary PSURs and Maximum Residue Limit (MRL) from 23 May 2016. A Statement of Intent on the extended use of XML delivery files for all submissions via the eSubmission Gateway/Web Client is now available here.
29-02-2016
The eSubmission Change Management Board and the Human Harmonisation Maintenance Group (HHMG) have adopted the updated eCTD validation criteria. The eCTD criteria have been updated in line with published EU Module 1 v3.0 following public consultation. The NeeS validation criteria have been updated to align to the updated eCTD validation criteria. The eCTD validation criteria v6.0, the NeeS validation criteria v4.1 and related release notes are available here.
If you have any questions regarding further development or require clarification on these documents, please contact: EUM1spec@ema.europa.eu
23-02-2016
Version 01.19.0.2 of the eAFs has been made available to fix technical issue related to the locking of the variation form and issues with the availability of the controlled terminologies in all forms. If you have already started to fill in version 1.19.0.1 of the variation form please import the user filled data to the new version to ensure that the variation form is fully locked. This can be done using the functionality to export the user filled data from 1.19.0.1 and import it into 1.19.0.2. The updated release notes and the forms are now available here.
12-02-2016
As of the 11 February 2016 all ongoing procedures with PSURs available in the PSUR Repository will “switch-on”.
This covers all procedures, whether previously in the pilot or not. The circulation of assessment reports and comments via email is no longer in place and the PSUR Repository enhanced notification system will alert National Competent Authorities (NCAs) to document uploads into the system. The enhanced notifications contain relevant information which was originally circulated by assessment teams by email. All procedures starting from February onwards will run in switch-on mode only.
During the switch-on phase the EMA will also manually upload all the PSURs that are received by NCAs only, during the so called “reconciliation process”. This process applies to nationally authorised medicines only. The manual upload is the second pillar of the “switch-on” phase as it will allow the system to host full data sets for all procedures, thereby allowing the NCAs to fully test complete download capability. In order to support this phase of the project, and in addition gain further experience with the system, MAHs are strongly encouraged to submit all PSURs and supplementary information for nationally authorised medicines to the PSUR repository in addition to the submissions to the relevant NCAs. This will allow the EMA to rely less on the reconciliation process with the Member States, ensuring timely inclusion of PSURs in the system and better adherence to the procedural timetable; ultimately ensuring that no PSUR is left unassessed or assessed late.
Please be reminded that for centrally authorised medicines submission to the PSUR Repository is already mandatory. MAHs for nationally authorised medicines are also reminded that PSUR submissions both to the PSUR Repository and NCAs must be structured electronic submissions, i.e. eCTD or NeeS. PSURs submitted as pdf documents cannot be uploaded into the PSUR Repository, and will entail follow up with the relevant MAH.
The PSUR Repository will become mandatory on the 13 June 2016; from this moment on all PSUR submissions across Europe will be submitted to the EMA only via this channel.
Users should continue to report any issues they may have with the system through the PSUR Repository mailbox psurrepository@ema.europa.eu .
05-02-2016
A webinar on the Introduction to VNeeS 2.4 will be held on 24 February 2016 from 10.00 to 11.00 UK time, in collaboration with Topra.This webinar is dedicated to Industry.
This session will focus on changes in VNeeS 2.4 which will come into force on 1 July 2016, its rationale and impact on applicants. There will also be an opportunity to address other vet-specific queries relating to eSubmissions.
For more information and to register, please visit the Topra webinars webpage.
01-02-2016
As of today, 1 February 2016 the EMA is introducing a new Service Desk portal (https://servicedesk.ema.europa.eu) through which users can raise IT system related questions, issues and requests for services. This portal will improve the efficiency and effectiveness of technical support by allowing users to report issues, track progress of their queries and obtain answers to frequently asked questions – all in one place.
This new web based portal replaces the eAF@ema.europa.eu email address which is currently used for all eAF related queries.
The new portal also replaces the gatewaysupport@ema.europa.eu mailbox which is used for all technical queries concerning Web Client/Gateway set-up, registration details or the transmission failures of files in the production or test environment.
During 1 month transitional period until 1 March 2016, both the old mailbox system and the Service Desk portal may be used, however, we strongly recommend the use of the Service Desk portal for all relevant queries. Until 1 March 2016, any requests submitted by email to the functional mailboxes above will be transferred by EMA to the EMA Service Desk portal on the requestor’s behalf. The requestor will receive an email with a reference number for their enquiry. Please see the EMA Service Desk portal presentation.
Things to remember as of 1 February 2016:
To use this new portal, you will need to register (a one-time, automated self-registration process) if you are not already registered for any of the Agency’s other systems (e.g. Eudralink, MMS, EudraCT);
All requests and issues in relation to the electronic Application Forms (eAF) will be dealt through this portal;
Relevant issues with eSubmission Gateway registration and transmission failures using the eSubmission Gateway will be dealt through this portal;
Any ongoing incidents or service requests logged before 1 February will be handled using the existing process i.e. you will not receive a new query number and credentials to access the self-service portal;
Instead of reporting issues/questions on the eAF via email as previously, please select ‘Report an Issue with business applications/software’ option from the service desk menu and select the relevant application/system from the menu e.g. ‘electronic Application Form – eAF’;
Emails to the above addresses will receive an automated response that include a link to the new portal, however the existing eAF@ema.europa.eu and Gatewaysupport@ema.europa.eu email addresses will continue to be monitored until 1 March 2016;
eCTD@ema.europa.eu and the eSubmission@ema.europa.eu as well as registration to the eSubmission Gateway remain as a separate processes for now. Queries related to eCTD submissions can continue to be sent to eCTD@ema.europa.eu. To register to use eSubmission Gateway/Web Client please follow the guidance on the eSubmission Gateway webpage;
PSURrepository@ema.europa.eu mailbox used for all PSUR Repository related business and procedural queries will also remain outside the service portal. All technical questions related to transmission failures via the eSubmission Gateway should be addressed to the service portal;
As of 1 March 2016, all enquiries you wish to raise related to the electronic Application Forms and the eSubmission Gateway support will have to be logged via the EMA Service Desk portal (https://servicedesk.ema.europa.eu).
27-01-2016
The PSUR Repository bulletin has been updated and can be found here.
25-01-2016
The use of eCTD format for centralised procedure human ASMF submissions will become mandatory from 1 July 2016. The Statement of Intent can be found here.
22-01-2016
Version 01.05 of the PSUR Repository was made available on 6th January 2016. This release is a major milestone in the PSUR Repository project marking the completion of the delivery of all post-audit functionalities in line with the plan approved by the EMA Management Board in June 2015. The updated system release notes can be found here.
Updated ‘util folder package’ reflecting the changes in the updated EU M1 v3.0 specification v.3.0 is now available here.
21-01-2016
The PSUR Repository bulletin has been updated and can be found here.
12-01-2016
Version 01.19.0.1 of the eAFs now replace versions 1.18 and 1.19.0.0. More information can be found here.
21-12-2015
- The new version of the Guideline on eSubmissions for Veterinary Products - version 2.4 together with eSubmission Validation checklist - version 2.4 and tools is published under the Current Guidance section here.The new VNeeS Guideline, checklist and checker will come into force on 01/07/2016.
16-12-2015
- The PSUR Repository bulletin has been updated and can be found here.
15-12-2015
- The updated eAF release schedule is now available and can be found here.
- Presentation and recording from the training session on eAF to Industry users held on 1 December 2015 is available here. Updated
- A second webinar on the eAFs (initial, variation, renewal) for Human and Veterinary procedures will be held on 11 January 2016 from 13.00 to 14.30 UK time. This webinar is dedicated to Industry.
This session will provide information where to find relevant documents and will address the most common issues and workaround solutions faced by industry when filling the forms. Please contact eAFwebinar-registration@ema.europa.eu by 6 January 2016 to register.
Should several participants from the same company wish to attend, it is advised to send only one registration request and attend from the same room.
The session will be recorded and posted on the eAF eSubmission website shortly after. If your registration for the session on 1 December 2015 has been unsuccessful, you will receive an invitation to this webinar automatically by 11 December 2015.
Please note: There will be no scope for live questions on 11 January 2016. If you have questions not addressed in the training session held on 1 December 2015, please email them to eAFwebinar-registration@ema.europa.eu by 6 January 2016.
- Recordings from the eAF webinar sessions with National Competent Authorities are now available for NCA users and can be found here.
25-11-2015
Important to Note for Friday, 27 November 2015, 07:00-09:00 hrs
- Due to essential maintenance to our systems, the PSUR Repository will be unavailable available on Friday, 27 November 2015, 07:00-09:00hrs. The NCA user interface will not be available during this period. The user interface for the generation of the XML delivery file will not be available during this period. The eSubmission Gateway will continue to be available throughout this period for sending previously created submissions.
23-11-2015
- A number of defects have been reported in the release v.1.19 of the eAFs. The EMA is working hard to fix these issues as soon as possible and a hotfix release will be deployed as soon as possible. Meanwhile if you are experiencing issues with any of the electronic application forms v.1.19 as published on 9th of November 2015 please inform the EMA as soon as possible via eAF@ema.europa.eu and continue using v.1.18 which remains available. The date of v 1.19 coming into force will be delayed to allow applicants who are experiencing issues with the new versions to continue submitting using v. 1.18. The new date of mandatory use of v. 1.19 will be published with the hotfix release of the forms.
20-11-2015
- The PSUR Repository bulletin has been updated and can be found here.
16-11-2015
- The eCTD validation criteria has been updated in line with published EU Module 1 v3.0 and has been released for public consultation until 14.December.2015. The validation criteria v.6.0 and related release notes and the comments template can be found here. The eSubmission Change Management Board and the Human Harmonisation Maintenance Group (HHMG) would like to invite all pharmaceutical companies, vendors of eCTD building and reviewing tools, and competent authorities to review and comment on the updated validation criteria. Please provide your comments, using the comments form, to EUM1spec@ema.europa.eu no later than 14 December 2015.
If you have any questions regarding further development or require clarification on these draft documents, please contact: EUM1spec@ema.europa.eu.
09-11-2015
- New versions of the electronic Application Forms (eAF v. 01.19) are now available and can be found here. These versions provide usability improvements to the existing forms and reflect the changes in the NtA application forms as published by the European Commission in September 2015. These versions replace the current version of eAF v. 01.18 after a transitional period of 1 month on 30 November 2015 and should be used after this date. The use of the eAFs is mandatory for all centralised procedure for human and veterinary submissions since 1 July 2015 and will become mandatory for all European procedures covered by the eAFs including Mutual Recognition, Decentralised Procedure and the national applications from 1 January 2016 as per the HMA eSubmission Roadmap.
- A webinar on the eAFs (initial, variation, renewal) for Human and Veterinary procedures will be held on 01 December 2015 from 13.00 to 14.30 UK time.
This webinar is dedicated to Industry.
This session will not demonstrate how to fill in the forms but will rather provide information where to find relevant documents and will address the most common issues and workaround solutions faced by Industry when filling the forms. It will also explain the support structure to be followed for business related queries for MRP/DCP/National applications and Centralised ones.
Please contact eAFwebinar-registration@ema.europa.eu by 26 November 2015 to register and to send any queries you would like to be addressed during this webinar.
Should several participants from the same company wish to attend, it is advised to send only one registration request and attend from the same room.
The session will be recorded and posted on the eAF eSubmission website shortly after.
03-11-2015
- The recording of the PSUR Repository v.1.04 training with the MAHs is now available along with the updated presentation from the follow-up interactive Q&A session here.
- The CAP Dossier Requirements document has been updated and is available here.
27-10-2015
- The Annex I – Guide to EMA filenaming convention for eSubmission document has been updated and can be found here.
- The PSUR Submission requirements during the transitional period document has been updated and can be found here.
21-10-2015
- The PSUR Repository bulletin has been updated and can be found here.
19-10-2015
- Version 3.0 of the EU Module 1 eCTD v.3.2.2 specification is now available. This EU M1 update sees an introduction of a new submission attribute containing a Universally Unique Identifier (UUID), introduction of the concept of ‘submission unit’ as well as a number of new regulatory activities among other updates. Details of the changes can be found in the Release notes. The updated EU M1 Specification and related Annex can be found here. The Human Harmonisation Group would like to thank all parties for input and comments to the draft specification.
- The eAF Technical Guidance document and the Q&A document have been updated and are now available here. Further updates will be provided to both documents following the release of the new version of the eAFs.
14-10-2015
- Version 01.04 of the PSUR Repository is available for both MAHs and NCAs. This new release provides change requests further enhancing the system for example the completely new product selection functionality for MAHs in the delivery file creation screen and the deduplication functionality for NCAs. The release also provides fixes for defects that have been identified during the pilot phase. The updated system release notes and MAH and NCA user guides can be found here.
09-10-2015
- The PSUR Repository bulletin has been updated and can be found here.
05-10-2015
- Annex VI to the HMA eSubmission Roadmap on the implementation of mandatory VNeeS format for Veterinary regulatory submissions document is available here.
- Training sessions and a follow-on interactive Q&A sessions for MAHs and other industry users as well as NCA (member states) users concentrating on the new features of the PSUR repository v1.04.00 will be organised in October 2015. These sessions will be recorded and the recordings will be made available on the PSUR Repository webpage. Dates and times for these sessions and more information about the sessions can be found here. Email PSURrepository@ema.europa.eu to book your place.
17-09-2015
- Forthcoming User Acceptance Testing (UAT) of version 1.19 of the eAFs. This will be the last UAT before the use of the eAF becomes mandatory for all EU procedures (vet and human) from January 2016, so it is important that participation is high in order to ensure that all remaining defects are identified and fixed and any change requests are prioritised appropriately. Please see the eAF page for more information.
- The PSUR Repository bulletin has been updated and can be found here.
01-09-2015
- From 1 September 2015 all PSUR submissions via the eSubmission Gateway / Web Client should be submitted with an xml delivery file. It will no longer be possible to use filenaming conventions for PSUR submissions in future. Annex 1 describing the detailed filenaming conventions for all eSubmission Gateway / Web Client submissions has been updated and can be found from the eSubmission Gateway / Web Client webpage.
14-08-2015
- PSUR recorded webinar training sessions and updated presentation slides for Marketing Authorisation Holders and National Competent Authorities users are available here.
06-08-2015
- Version 01.03 of the PSUR Repository is now available for both MAHs and NCAs. This new release provides change requests further enhancing the system and fixes defects identified during the pilot phase. The updated system release notes and MAH and NCA user guides can be found here.
- The PSUR Repository bulletin has been updated and can be found here.
03-08-2015
- Release 01.18 of the electronic Application Forms (eAF v. 01.18) now replaces the previous forms. These new versions provide usability improvements and improved functionality. The eAFs and all supporting documents can be found here. The use of the eAFs is mandatory for all centralised procedure human and veterinary submissions since 1 July 2015 and will become mandatory for all other procedures (MRP/DCP/National) from January 2016 as per the HMA eSubmission Roadmap.
24-07-2015
- The Common Repository Q&A document has been updated and can be found here.
- Training sessions and a follow-on interactive Q&A sessions for MAHs and other industry users as well as NCA users concentrating on the new features of the PSUR repository v1.03.00 will be organised in August and September 2015. These sessions will be recorded and the recordings will be made available on the PSUR Repository webpage. Dates and times for these sessions and more information about the sessions can be found here. Email PSURrepository@ema.europa.euto book your place.
21-07-2015
- The eCTD EU Module 1 v3.0 has been released for public consultation until 14.9.2015 and the updated version can be found here. The eSubmission Change Management Board and the Human Harmonisation Maintenance Group (HHMG) invites all pharmaceutical companies, vendors of eCTD building and reviewing tools, and competent authorities to send comments. Please complete the relevant tab(s) on the comments form and return it to EUM1spec@ema.europa.eu no later than 14 September 2015.
If you have any questions regarding further development or require clarification on these draft documents, please contact: EUM1spec@ema.europa.eu.
13-07-2015
- The User Guides for the electronic Application Forms for Marketing Authorisation for Human and Veterinary use have been updated and are now available on the CMDh website and CMDv website and can also be found here.
06-07-2015
- New versions of all electronic Application Forms (eAF v. 01.18) are now available and can be found here. These versions provide usability improvements to the existing forms. The new versions of the forms replace the current version of eAF v. 01.17 after a transitional period of 1 month on 3 August 2015 and should be used this date. The use of the eAFs is now mandatory for all centralised procedure human and veterinary submissions since 1 July 2015 as per the HMA eSubmission Roadmap.
01-07-2015
- The use of the Common Repository for Human Centralised Procedure submissions is now mandatory for all National Competent Authorities. Submissions sent to EMA via eSubmission Gateway/Web Client will be considered delivered to all National Competent Authorities’ representatives and alternates. Submissions for Centralised Procedure should be made via EMA eSubmission Gateway/Web Client only. Additional copies should not be submitted directly to the NCAs on CD/DVD or via CESP as this might lead to validation issues and cause delays. More information on the Common Repository can be found here.
- The use of the electronic Application Forms (eAF) is now mandatory for all Human and Veterinary Centralised Procedure submissions. The forms for Initial, variation and renewal applications as well as a User Guidance document and an updated Q&A document are also available on the eAF webpage.
23-06-2015
- On 11 June 2015 the EMA Management Board, based on a positive PRAC Recommendation and an independent audit report, announced that the PSUR repository meets the functional specifications as agreed in the ‘PSUR Repository functionalities to be audited’ document and concluded that it has achieved its full functionality. The legislation foresees that 12 months after the EMA Management Board announcement , the use of the repository for the submission, storage and retrieval of all PSURs and related documents (assessment reports) in the European Union will become mandatory (13 June 2016).
Full news item can be found on the EMA public website
- The scope for release v.1.03.00 has been agreed, and scheduled for early August 2015. The NCA and Industry user acceptance testing (UAT) will take place between the 17th and 23rd July. NCAs and industry representatives interested in participating in the UAT should contact Turi Salami (turi.salami@ext.ema.europa.eu) by the 25th June.
The main enhancements provided in this version are:
- Improvements to the product search functionality;
- Additional search criteria for PSURs and supplemental information within the non-EU Single assessment screen;
- Addition of national authorisation numbers to facilitate searches when uploading Non EU single assessment documents, and
- The addition of information regarding silent adoption/plenary discussion in the assessment report upload screen.
The “Kick-off” meeting with PSUR Repository Advisory Group to prepare the UAT will take place on the 26th June 2015.
10-06-2015
- France has joined the Common Repository to receive Centralised Procedure submissions on 3 June 2015. The large majority of the NCAs are now using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. In compliance with the HMA eSubmission Roadmap, it will be mandatory for all NCAs to use the Common Repository from 1st July 2015. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated.
- Annex III to the HMA eSubmission Roadmap on Transitioning to the mandatory use of the Common Repository for eCTD format Centralised Procedure submissions document is available here.
08-06-2015
- The PSUR Repository bulletin has been updated and can be found here.
01-06-2015
-
Version 01.02 of the PSUR Repository is now available. This release provides change requests enhancing the system and fixes defects since the start of the pilot phase. In particular, this release provides solutions for all remaining findings from the audit carried out by PwC Luxembourg in Feb-Mar 15.
-
Industry associations and NCAs are invited to take part in a User Acceptance Testing of the eAF v.01.18. The industry testing will take place 1.-5.6.2015 and the NCA testing will take place 8.-12.6.2015. If you would like to participate in the UAT, please contact Turi.Salami@ext.ema.europa.eu
-
New versions of the eAF DES and schemas have been released and can be found here.
-
The use of the eAFs will become mandatory for all Centralised procedure Initial Marketing authorisation applications, Variations and Renewals from 1 July 2015. More information on the mandatory use can be found here.
28-05-2015
The PSUR Repository Project team organised a Q&A webinar on 21 May to assist NCAs in preparing for usage of the API for automated exchange of information between the NCA IT systems and the PSUR Repository. Both IT aspects and business process aspects were addressed. As the overall feedback was that this initiative was useful, similar webinars will be organised in the near future. The slides of the webinar and the Q&A can be found here.
22-05-2015
- Due to essential maintenance to our systems, the PSUR Repository will not be fully available on Sat 23 May 15 09:00-15:00
Due to essential maintenance to our systems, the PSUR Repository will not be fully available on Sat 23 May 15 09:00-15:00.
The impact of this will be:
- The NCA user interface will not be available during that period.
- The user interface for generation of the XML delivery file will not be available during that period.
- The eSubmission Gateway will continue to be available throughout this period so MAHs can send PSUR submissions.
- An eAF benchmark response times document is now available and can be found here.
22-04-2015
- Draft specifications of the PSUR Repository API and Supplementary Specification are available here.
16-04-2015
- A new PSUR Bulletin is now available and can be found here.
13-04-2015
- The meetings page has been updated and can be viewed here.
31-03-2015
- The Guideline on eSubmissions for Veterinary Products - version 2.3 has been updated and can be found here.
- The eSubmission Validation checklist - version 2.3 has been updated and can be found here.
- The Change request tracking table has been updated and can be viewed here.
24-03-2015
- Version 01.01 of the PSUR Repository is now available. This release provides change requests enhancing the system and fixes defects identified after the UAT end 2014 or since the start of the pilot phase
23-03-2015
20-03-2015
- PSUR Repository version 01.01 will be available for MAH and NCAs on 24 March 2015. This release provides change requests enhancing the system and fixes defects identified after the UAT or since the start of the pilot phase. The updated system release notes and links to both, MAH and NCA user interfaces can be found here.
- The use of the XML delivery file for all PSUR submissions via the eSubmission Gateway/Web Client will become mandatory from 1 September 2015. Please see the statement of intent here.
- The new versions of all electronic Application Forms (eAF) will be available on 23 March 2015 and can be found here. The new versions provide improvements to the existing forms in preparation for the mandatory use of the eAFs for all centralised procedure human and veterinary submissions from 1 July 2015 as per the HMA eSubmission Roadmap and the related annex to the roadmap describing the steps to the mandatory use.
- A new PSUR Bulletin is now available and can be found here.
17-03-2015
- Please Note: eAF will be unavailable on Monday, 23rd March 2015 from approx. 07.00 to 07.30 GMT. We apologise for any inconvenience this may cause.
12-03-2015
- The eCTD version 4.0 EU M1 Implementation Guide is now available for public consultation. The eSubmission Change Management Board invites all pharmaceutical companies, vendors of eCTD building and reviewing tools and competent authorities to take part in this consultation and provide comments by 22 May 2015. Please provide your comments using the comment template; and any questions regarding further development and clarifications of the released draft documents to: eCTD4consultation@ema.europa.eu. The Implementation Guide and relevant documents can be found here.
27-02-2015
- A new PSUR Bulletin is now available and can be found here.
- Interactive Q&A sessions are being organised by the PSUR Repository team to support the use of PSUR Repository in the view of the ongoing pilot. There will be a short demo of the system after which we will answer your questions about the repository. In the NCA session there will be a short demo of the search and retrieval functionality as well as how to submit your assessment reports and comments to the repository. After the demo NCAs will have an opportunity to ask questions on the use of the repository. Please see here to book your place.
- Updated Variations in eCTD format Q&A document has been updated by the Harmonisation Group and can be found here.
20-02-2015
- The next version of the eAFs is now available for User Acceptance Testing until 5th of March 2015 for limited number of testers. If you are interested to participate in this exercise, please see more information from here
- Recordings from the webinar trainings on the use of PSUR Repository for new and existing Gateway users and recording from the MAH pilot training are now available here
16-02-2015
- New versions of the eAF DES and schemas have been released and can be found here.
13-02-2015
- A new PSUR Bulletin is now available and can be found here.
- Presentations from the webinar training sessions for existing and new Gateway users have been updated and are available here.
- Annex V to the HMA eSubmission Roadmap on the implementation of mandatory use of electronic application forms (eAFs) is now available and can be found here.
10-02-2015
- A communication leaflet concerning the mandatory use of the eAF has been published and can be found here.
09-02-2015 - Please Note
- eAF will be unavailable on Wednesday 11th February 2015 from approx. 17.30 to 18.30 GMT. We apologise for any inconvenience caused.
06-02-2015
- A new PSUR Bulletin is now available and can be found here.
02-02-2015
- The EMA PSUR Repository team is introducing a regular news bulletin with the intention of keeping the EU Regulatory Network and Industry up to date with the most relevant repository related activities and milestones.Our intention is to facilitate continuous engagement and enhance the dialogue and collaboration between stakeholders and the project. The new bulletin is available here.
- Updated Annex II to the HMA eSubmission Roadmap on the implementation of mandatory eCTD format for regulatory submissions is now available here.
29-01-2015
- Please Note Due to essential maintenance to our database systems, the PSUR Repository will not be available on Sat 31 Jan 15 09:00-18:00. The eSubmission Gateway will continue to be available throughout this period so MAHs can send PSUR submissions.
26-01-2015
- The PSUR Repository is available and it is now possible to submit PSURs to the PSUR Repository. The delivery files required for the submissions can be created here. The system is also available to all registered NCAs for pilot use here. More information on the PSUR Repository can be found from the PSUR Repository page here.
20-01-2015
- Annex I to the HMA eSubmission Roadmap on the implementation of eCTD version 4.0 and Annex II to the HMA eSubmission Roadmap on the implementation of mandatory eCTD format for regulatory submissions is now available and can be found here
23-12-2014
- Romania will join the Common Repository to receive Centralised Procedure submissions from 1st January 2015. The majority of the NCAs are now using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. In compliance with the HMA eSubmission Roadmap, it will be mandatory for all NCAs to use the Common Repository from 1st July 2015. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated
10-12-2014
- A dedicated webpage for PSUR Repository is now available here. News and announcements related to the system availability including all user guidance documents will be published on this page.
26-11-2014
Malta has now joined the Common Repository to receive Centralised Procedure submissions. 18 NCAs are now using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. In compliance with the HMA eSubmission Roadmap, it will be mandatory for all NCAs to use the Common Repository from 1st July 2015. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated
21-11-2014
HMA eSubmission Roadmap
The Final HMA eSubmission Roadmap was adopted by the EUTMB on 1st of October 2014. The common agreed vision for the objectives on eSubmissions in Europe are outlined in the eSubmission Roadmap which can be found here
27-10-2014
eAF
The eAF term request process has been updated and the relevant documents can be found here
Veterinary Submissions
The filenaming conventions for Veterinary Submissions have been simplified. It is now easier to submit Veterinary submissions using the eSubmission Gateway and the Web Client. Updated Annex 2 can be found here
Deadline approaching - Referral Procedures to be sent via eSubmission Gateway / Web Client from 1st of November 2014
From 1 November 2014 all submissions for Referral Procedures for human medicinal products should be sent via the eSubmission Gateway or the Web Client. After 1 November 2014, the EMA is no longer accepting electronic submissions for referrals on CD or DVD.
The European Medicines Agency (EMA) strongly recommends using the electronic submission channels (eSubmission Gateway or Web Client) and the electronic Common Technical Document (eCTD) or NeeS (Non-eCTD electronic Submission) formats for submission of referrals. For Referral submissions related to Centrally Authorised products, it is mandatory to use the eCTD format.
The EMA's strategy for the electronic submission of applications aims to improve efficiency and decrease costs for applicants. Applicants who wish to use the eSubmission Gateway or Web Client are encouraged to register to use the eSubmission Gateway/Web Client on the EMA’s eSubmission Gateway website as soon as possible.
13-10-2014
Portugal has now joined the Common Repository to receive Centralised Procedure submissions. More than half of the NCAs are now using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. In compliance with the HMA eSubmission Roadmap, it will be mandatory for all NCAs to use the Common Repository from 1st July 2015. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated
08-10-2014
Hungary has now joined the Common Repository to receive Centralised Procedure submissions. More than half of the NCAs are now using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. In compliance with the HMA eSubmission Roadmap, it will be mandatory for all NCAs to use the Common Repository from 1st July 2015. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated.
02-10-2014
eAF 1.16.0.1 hot fix has been deployed and can be found here
26-09-2014
New versions of the eAF forms, release notes, DES and schemas have been released, and can be found here
11-09-2014
Italy and Spain have now joined the Common Repository to receive Centralised Procedure submissions. There are currently 15 NCAs that are using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated
01-09-2014
Regulatory information - Referral Procedures to be sent via eSubmission Gateway / Web Client from 1st of November 2014
From 1 November 2014 all submissions for Referral Procedures for human medicinal products should be sent via the eSubmission Gateway or the Web Client. After 1 November 2014, the EMA is no longer accepting electronic submissions for referrals on CD or DVD.
The European Medicines Agency (EMA) strongly recommends using the electronic submission channels (eSubmission Gateway or Web Client) and the electronic Common Technical Document (eCTD) or NeeS (Non-eCTD electronic Submission) formats for submission of referrals. For Referral submissions related to Centrally Authorised products, it is mandatory to use the eCTD format.
The EMA's strategy for the electronic submission of applications aims to improve efficiency and decrease costs for applicants. Applicants who wish to use the eSubmission Gateway or Web Client need to register on the EMA’s eSubmission Gateway website.
22-08-2014
Norway has now joined the Common Repository to receive Centralised Procedure submissions. There are currently 13 NCAs that are using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated.
And
New versions of the eAF DES and schemas have been released and can be found here
14-08-2014
Bulgaria, Czech Republic and Luxembourg have now joined the Common Repository to receive Centralised Procedure submissions. There are currently 12 NCAs that are using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. Please see details on the Common Repository page. The CAP Dossier Requirements document has been updated
01-08-2014
Belgium, Greece and Slovakia have now joined the Common Repository to receive Centralised Procedure submissions. There are currently 9 NCAs using the Common Repository as their only source to receive Centralised Procedure eCTD submissions. Please see details on the Common Repository page. Additionally the CAP Dossier Requirements document has been updated.
And
A further draft of the eSubmission Roadmap v.1.0 has now been endorsed by the IT Directors Executive Committee on 24 July 14 and will be on the agenda for the next EUTMB for further endorsement. More details on the implementation of the Roadmap will also be published at a later stage by the eSubmission CMB.
And
The European Medicines Agency has moved. Our new address is:
Domenico Scarlattilaan 6 | 1083 HS Amsterdam | London E14 5EU | United Kingdom
09-07-2014
Croatia is now using the Common Repository to receive Centralised Procedure submissions. The CAP Dossier Requirements document has been updated
As of 1.2.2020, the UK is no longer an EU Member State. However, EU law still applies to the UK during the transition period.
|



