|
The Common Technical Document (CTD) describes the organisation of modules, sections and documents to be used by an Applicant for a Marketing Authorisation for a medicinal product for human use agreed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). The electronic Common Technical Document (eCTD) allows for the electronic submission of the Common Technical Document (CTD) from applicant to regulator. While the table of content is consistent with the harmonised CTD, the eCTD also provides a harmonised technical solution to implementing the CTD electronically. In other words, an eCTD is the submission of PDF documents, stored in the eCTD directory structure, accessed through the XML backbone and with the files integrity guaranteed by the MD5 Checksum.
The current version of the eCTD specification to be used for CTD modules 2-5 is the Electronic Common Technical Document Specification V3.2.2 (PDF). More information about the standard can be found at the ICH eCTD webpage.
For eCTD submissions within EU, the EU Module 1 eCTD Specification (see link below) should be used.
The eCTD format is mandatory to use for all submission types related to Marketing Authorisation for products within all EU procedures (i.e. Centralised, Decentralised and Mutual Recognition Procedures). In accordance with the eSubmission Roadmap, Mandatory eCTD format is also stepwise introduced for National Procedures.
28-11-2025
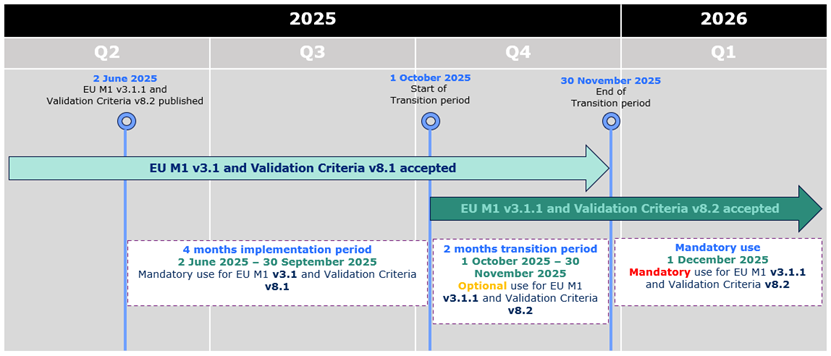
eCTD 3.2.2 - EU M1 v3.1.1 and Validation Criteria v8.2
mandatory from 1st December
As previously announced, the updated EU M1 specification v3.1.1 and the
related Validation Criteria v8.2 were accepted from 1st
October 2025, and
the mandatory use of the updated specification and validation criteria will
commence on 1st December 2025.
The changes are reflected in the Release Notes.
16-09-2025
eCTD 3.2.2 - EU M1 v3.1.1 and Validation Criteria v8.2 accepted from 1st October
As previously announced, the updated EU M1 specification v3.1.1 and the related
Validation Criteria v8.2 are accepted from 1st October 2025 kicking
off 2 months transitional period during which both the current and the new specification and
validation criteria can be used. The mandatory use of the updated specification
and validation criteria will commence on 1st December 2025.
The changes are reflected in the Release Notes.
02-06-2025
eCTD 3.2.2 - new package available
A new version of the
EU eCTD M1 Specification, version 3.1.1
,
is now published on the
eSubmission website
.
The version 3.1.1 sees the introduction of a small number of changes in the
specification, related to the tracking table for EDQM and new examples of product
numbers and procedure numbers for work-sharing and super-grouping.
The changes are reflected in the
Release Notes
.
A new version of the
validation criteria v8.2
has been published on the
eSubmission website. The version is related to the
EU Module 1 Specification version 3.1.1 and should be used in case of submitting a new
sequence according to EU M1 specification v3.1.1. The new validation criteria will
be used for the technical validation for all v3.1.1 electronic submissions received
as of 1 December 2025 to the NCAs and EMA.
The changes are reflected in the
Release Notes.
A new version of the
Harmonised guidance
eCTD 6.0.1 was published together with the new version of the specification and the validation criteria
Note: The Util files remain the same
During the initial period of 4 months from 2 June 2025 to 30 September 2025,
applicants can only submit eCTD format submissions compliant with EU M1 v3.1 and validation criteria version 8.1.
From 1 October 2025 eCTDs compliant with EU M1 v3.1 or v3.1.1 and validation criteria v8.1 or v8.2 are accepted.
From 1 December 2025 only eCTDs compliant with EU M1 v3.1.1 and validation criteria v8.2 are accepted.
10-12-2024
eSubmissions Gateway – naming of the working documents folder
When submitted with an eCTD, the Working Documents should always be provided in a separate folder called "xxxx-workingdocuments" on the same submission zip package containing the eCTD, where the number (xxxx) matches the number of the eCTD sequence being submitted.
Any deviation will lead to a failed submission, and the package will have to be resubmitted with the correct naming. For example, if sending sequence "0007", which contains working documents, the separate folder should be named "0007-workingdocuments".
More details can be found in chapter 2.9.10 of the Harmonised guidance eCTD - version 6.0.
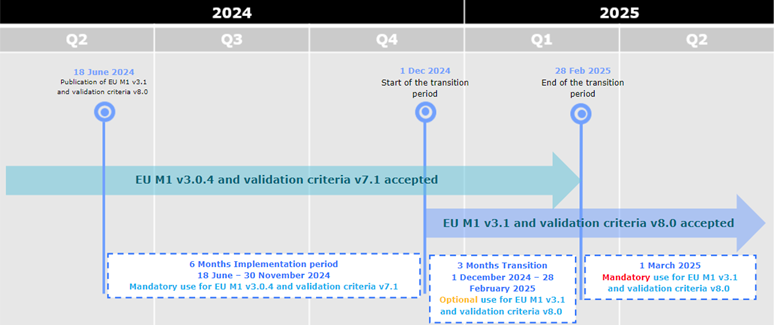
05-12-2024
EU Harmonised technical eCTD guidance version 6.0 now available
A new updated version of the eCTD EU Harmonised technical guidance is now available
here, together with the Release notes.
The EU Harmonised technical guidance is aligned with the EU M1 eCTD Specification v3.1 and the Validation Criteria v8.1.
The timeline for implementation is as follows:
- (optional use): From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and validation criteria v7.1 or v8.1 are accepted.
- (mandatory use): From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.1 are accepted.
28-10-2024
Minor update to the published EU Validation criteria
An updated version of the validation criteria to add further
clarification the has been published on the eSubmission
website. The version is related to the EU Module 1 Specification version 3.1 and should be
used in case of submitting a new sequence according to EU M1 specification v3.1. The new validation
criteria will be used for the technical validation for all v3.1 electronic submissions received
as of 1 March 2025 to the NCAs and EMA. The changes are reflected in the Release
Notes.
From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and
validation criteria v7.1, 8.0 or v8.1 are accepted.
From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.1
are accepted.
18-06-2024
eCTD 3.2.2 new package available
A new version of the EU eCTD M1 Specification, version 3.1, is now published on the eSubmission website
The version 3.1 sees the introduction of a number of changes across the specification, however, more specifically, the introduction of a new annex detailing the list of accepted file formats
(Reminder: the generally accepted file format is the PDF, however, in specific cases, other formats will be exceptionally accepted in the eCTD modules). The changes are reflected in the Release Notes.
The same changes are reflected in the additional files of the EU M1 Implementation Guide package (i.e. eu-envelope.mod, eu-regional.dtd, eu-regional.xsl)
A new version of the validation criteria has been published on the eSubmission website. The version is related to the EU Module 1 Specification version 3.1 and should be used in case of submitting a new sequence according to EU M1 specification v3.1. The new validation criteria will be used for the technical validation for all v3.1 electronic submissions received as of 1 March 2025 to the NCAs and EMA. The changes are reflected in the Release Notes.
During the initial period of 6 months from 18 June 2024 to 30 November 2024, applicants can only submit eCTD format submissions compliant with EU M1 v3.0.4 and validation criteria version 7.1.
From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and validation criteria v7.1 or v8.0 are accepted.
From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.0 are accepted.
Documentation
NeeS format
NeeS has been used as a transitional format towards the mandatory use of eCTD and is now only accepted in some specific cases.
For the UK, as from 1.1.2021, EU Law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland/NI. |

