|
Previous PSUR Bulletins
Week commencing 04 April 2016
- The mandatory use deadline of the PSUR Repository is fast approaching (13 June 2016) at which time all PSUR submissions within the EU should be centralised through the PSUR Repository. To this effect NCAs, Industry and EMA should ensure business processes are ready for this new phase.
- The last release of the PSUR Repository (v.1.06.00) is scheduled for the 14 May 2016 and is currently in the user acceptance testing (UAT) phase. The EMA user acceptance testing (UAT) started on the 30 March and was finalised yesterday 5 April. The NCA/Industry UAT will take place between the 18 April – 22 April. NCAs and Industry representatives interested in participating in the UAT should contact Turi Salami (turi.salami@ext.ema.europa.eu).
The main enhancements provided in this version are:
- Ability for NCAs to indicate lifecycle invalid eCTD submissions in the PSUR repository with automated message to the MAH
- Provide the functionality to create a unique name for each of the XML delivery file
- Ability for MAH to create xml delivery file and submit after submission deadline with ‘late submission id’ provided by the EMA upon request
- Improved/amended notifications
- No need to manually enter the EMA product number for CAPs in the delivery file
- No need to manually enter the sender and receiver routing IDs in the delivery file
With release v.1.06.00 the project will have completed the delivery of all the agreed functionality and the system will be in full production. This milestone will mark the transition of the PSUR Repository project into its maintenance phase with a subsequent change of the project governance structure. The EMA Management Board agreed to this transition in its March 2016 meeting. The operational aspects regarding this transition are currently under discussion with the members of the PSUR Advisory group, to ensure a smooth transition into the new model of working.
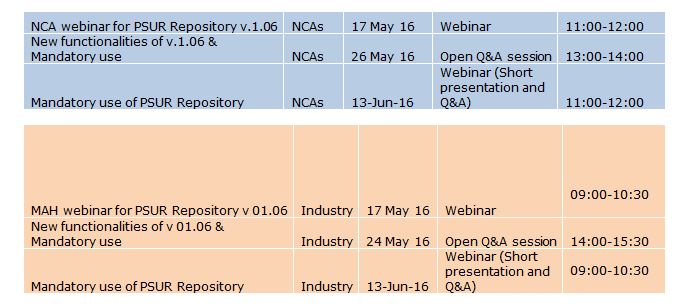
- A series of trainings and Q&As have been scheduled in preparation both for the upcoming release v.1.06.00 and for the mandatory use (see tables below). NCAs and Industry representatives should take note of these dates, NCA representatives will receive an invitation from EMA. The industry representatives are encouraged to register for the training by contacting Turi Salami (turi.salami@ext.ema.europa.eu). These training and Q&A sessions will be recorded and the recordings will made available on the eSubmissions PSUR Repository page.
- All MAHs are strongly encouraged to submit all PSURs and supplementary information for nationally authorised medicines to the PSUR repository in addition to the submissions to the relevant NCAs. Please be reminded that for centrally authorised medicines submission to the PSUR Repository is already mandatory. MAHs are also reminded that PSUR submissions both to the PSUR Repository and NCAs must be structured electronic submissions, i.e. eCTD or NeeS.
As of the 11 February 2016 all ongoing procedures with PSURs available in the PSUR Repository will “switch-on”.
This covers all procedures, whether previously in the pilot or not. The circulation of assessment reports and comments via email is no longer in place and the PSUR Repository enhanced notification system will alert National Competent Authorities (NCAs) to document uploads into the system. The enhanced notifications contain relevant information which was originally circulated by assessment teams by email. All procedures starting from February onwards will run in switch-on mode only.
During the switch-on phase the EMA will also manually upload all the PSURs that are received by NCAs only, during the so called “reconciliation process”. This process applies to nationally authorised medicines only. The manual upload is the second pillar of the “switch-on” phase as it will allow the system to host full data sets for all procedures, thereby allowing the NCAs to fully test complete download capability. In order to support this phase of the project, and in addition gain further experience with the system, MAHs are strongly encouraged to submit all PSURs and supplementary information for nationally authorised medicines to the PSUR repository in addition to the submissions to the relevant NCAs. This will allow the EMA to rely less on the reconciliation process with the Memberuro States, ensuring timely inclusion of PSURs in the system and better adherence to the procedural timetable; ultimately ensuring that no PSUR is left unassessed or assessed late.
Please be reminded that for centrally authorised medicines submission to the PSUR Repository is already mandatory. MAHs for nationally authorised medicines are also reminded that PSUR submissions both to the PSUR Repository and NCAs must be structured electronic submissions, i.e. eCTD or NeeS. PSURs submitted as pdf documents cannot be uploaded into the PSUR Repository, and will entail follow up with the relevant MAH.
The PSUR Repository will become mandatory on the 13 June 2016; from this moment on all PSUR submissions across Epe will be submitted to the EMA only via this channel.
Users should continue to report any issues they may have with the system through the PSUR Repository mailbox psurrepository@ema.europa.eu .
Week commencing 18 January 2016
- We are happy to announce the positive outcome of the written consultation with the EU Regulatory Network on the move to the switch-on phase of the PSUR Repository project, with no blocking issues identified by the Member States.
- Switch-on entails that all PSURs for PSUSA procedures will be available via the Repository as the EMA will pro-actively upload submissions made to NCAs only following reconciliation. The National Competent Authorities (NCAs) will continue receiving nationally authorised product (NAP) PSURs locally until the mandatory use (June 2016), hence the download function is optional (e.g. existing automated downloads from CESP remain available).
The key difference is the use of the enhanced notification system instead of Eudranet emails, and the upload of assessment reports and comments via the repository without the circulation of manually drafted emails to the functional mailboxes.
The switch-on phase will allow for further learning and preparation for the mandatory use of the system, and allow for optimal prioritisation of existing change requests.
- As of the 11 February 2016 all ongoing procedures with PSURs available in the PSUR Repository will “switch-on”. This covers all procedures, whether previously in the pilot or not. The circulation of assessment reports and comments via email will stop and the PSUR Repository enhanced notification system will alert NCAs to uploads into the system. The enhanced notifications contain information which was originally circulated by assessment teams by email.
- All procedures starting from February onwards will run in switch-on mode only.
- In the view of the upcoming switch-on phase all MAHs are strongly encouraged to submit all PSURs and supplementary information for nationally authorised medicines to the PSUR repository in addition to the submissions to the relevant NCAs. Please be reminded that for centrally authorised medicines submission to the PSUR Repository is already mandatory.
- We would like to remind MAHs that the issue related to the product selection at the time of the XML delivery file creation is fully resolved. It is important that all PSUR submissions to the EMA are sent to the repository using the xml delivery file and that the workaround solutions used previously are no longer applied.
- Users should continue to report any issues they may have with the system through the PSUR Repository mailbox psurrepository@ema.europa.eu .
Week Commencing 11 January 2016
- We are happy to announce that release of version 1.05.00 of the PSUR Repository went live on the 6th of January 2016. This release is a major milestone in the PSUR Repository project marking the completion of the delivery of all the post-audit functionalities in line with the plan approved by the EMA Management Board in June 2015. The project now enters into the final months before the agreed mandatory use of the system both for Industry and for the EU Regulatory Network. The mandatory use will commence on the 13 June 2016.
- A dedicated Interactive Q&A session with NCAS on the use of PSUR repository v 01.05.00 is scheduled for the 19 January 12:00 – 13:00 (UK time). The purpose of this session is to ensure that all NCAs are familiar with the new notification system and availability of the assessment report template via the repository. This session will be recorded and the recording will be made available via the eSubmission webpage.
- With the full delivery of the post-audit functionality the EMA has initiated discussions with the network on the closure of the project pilot phase and move to the switch-on phase, and to this effect has launched a written consultation requesting NCAs to identify any potential blocking issues to this new phase of the project. The switch-on phase of the project aims at creating a simulated mandatory use of system for NCAs and EMA, and entails that assessment reports and comments will no longer be circulated by email. These documents will need to be accessed through the system and all activity will be supported by the recent delivery of the enhanced notification system of the repository. The switch-on is proposed from February 2016 onwards. National Competent Authorities are requested to submit any blocking issues to the switch-on by Friday, 22nd January, 15:00 (UK time).
- In the view of the upcoming switch-on phase all MAHs are strongly encouraged to submit all PSURs and supplementary information to the PSUR repository in addition to the submissions to the relevant NCAs.
- The scope for release v.1.06.00 is due to be confirmed on the 18 January, by the PSUR Repository Advisory Group, and the release is scheduled for the second quarter of 2016. The NCA and Industry user acceptance testing (UAT) will take place between the 18 April – 22 April, with a prior kick-off meet planned on the 18 March. NCAs and Industry representatives interested in participating in the UAT should contact Turi Salami (turi.salami@ext.ema.europa.eu).
The main enhancements proposed in this version are:
- Ability to remove wrongly submitted documents
- Provide the functionality to create a unique name for each of the XML delivery file
- Forwarding of assessment reports and recommendations to the MAH
- Improvement of EMA admin screen to make xml delivery file preparation easier or change the UI to allow the MAH to create the xml delivery file for submissions after the deadline (manual trigger required by EMA)
- Notifications amended to inform of procedure deadlines without any PSUR
- Prepare of delivery file to take the EMA product number from Art 57 instead of free text field.
- Improvement of the error handling and messages from the eSubmissions Gateway
- The issue related to the product selection at the time of the XML delivery file creation has now been solved for majority of procedures and the full functionality will be available in the system from Tuesday 19th January 12:00pm midday. This defect has been affecting only products which have been added or amended in the Art 57 database after the data lock point (DLP). It is important that all PSUR submissions are sent to the repository using the xml delivery file and workaround solutions used previously should no longer be used unless you are specifically requested to do so. If you continue to experience issues with the product selection, please report to PSURrepository@ema.europa.eu as soon as possible.
- Users should continue to report any issues they may have with the system through the PSUR Repository mailbox.
Week commencing 14 December 2015
- The UAT for release v.1.05.00 was closed on the 10 December 2015. No change requests were raised during the exercise, and the defects which were identified have been fixed. The release therefore remains on target for a deployment at the end of December and Go-Live in January 2016.
- This release is a major milestone in the PSUR Repository project marking the completion of post-audit functionalities in line with the plan approved by the EMA Management Board in June 2015. Confirmation of this milestone will be announced once achieved at the both PRAC and CMDh plenary meetings.
- The prioritisation exercise for v.1.06.00 is due to commence this week. The release is scheduled for Q2 2016 and will be the last planned release prior to the mandatory use of the repository (13 June 2016).
- The PSUR Repository continues to experience some issues in relation to the submission of PSURs which leads to some products being unavailable in the product selection when creating the XML delivery file. This defect affects only products which have been added or amended in the Art 57 database after the data lock point (DLP). The technical solution to the issue has already been discussed and agreed, and will be available in early 2016. In the interim MAHs have been provided with workaround solutions which enable the submission of PSURs to the EMA.
- Users should continue to report any issues they may have with the system through the PSUR Repository mailbox.
Week commencing 16 November 2015
- The UAT for release v.1.05.00 is scheduled to start on the 30 November 10:00 UK time with a dedicated session for the participants and will last until the 04 December. This UAT is only for NCAs as the release does not affect the functionality for MAHs. Interested parties should contact turi.salami@ext.ema.europa.eu.
- As soon as the UAT for v. 1.05.00 is finalised the prioritisation exercise for v.1.06.00 will be initiated in order to agree on the scope of this version. The release is scheduled for Q2 2016 and will be the last planned release prior to the mandatory use of the repository (13 June 2016).
- Important notice for MAHs: The PSUR Repository is currently experiencing some issues in relation to the submission of PSURs which leads to some products being unavailable in the product selection when creating the XML delivery file. This defect affects all products which have been added or amended in the Art 57 database after the data lock point (DLP). Unfortunately, due to this defect, the PSUR repository product selection listing cannot be refreshed overnight as usual. MAHs are recommended to use the following work-around solutions until the issue is resolved:
For CAPs and NAPs
- If some/any of the products that are covered in your PSUR document are available through the repository, we suggest that you create a delivery file and include all available products in that delivery file, and if possible, mention the issue in the cover letter. If you have already submitted the PSUR to the relevant NCAs, and hence it is not possible to modify the cover letter included in the package (the package to the NCAs and the EMA should be identical) inform EMA via PSURrepository@ema.europa.eu so that we can ensure that the product team is aware of the issue and can ensure that the missing products are included in the procedure scope.
For NAPs
Additionally please submit a convenience copy to the EMA PSURrepository@ema.europa.eu via Eudralink with accompanying justification for the reason why the submission is made via Eudralink. This is a very exceptional arrangement and sending submissions via Eudralink is normally not acceptable and submissions will be rejected/considered not received at the EMA.
For CAPs
- If none of your products are available, and you are unable to create xml delivery file please contact the EMA via the PSURrepository@ema.europa.eu mailbox to request a special filenaming convention for your submission. This is a very exceptional arrangement and sending PSUR and PSUR supplementary information submissions using filenaming convention is not acceptable without prior agreement with EMA and will lead to your submissions being rejected.
To avoid the above issues we recommend that MAHs review their Art 57 data PRIOR to the DLP deadline, in which case the product selection which not be impacted.
Users should continue to report any issues they may have with the system through the PSUR Repository mailbox.
- Week commencing 12 October 2015
- Release v.1.04.00 went live on the 14 October 2015 after the successful deployment of a component of the fees system which was needed to establish the link between the EURD list and Art 57.
- The UAT report for this release concluded that all of the functionality introduced in v1.4.0 PSUR Repository has passed UAT as per the previously agreed acceptance criteria.
- A dedicated Interactive Q&A session with NCAS on the use of PSUR repository v 01.04.00 is scheduled for the 23 October 12:00 – 13:30 (UK time). The purpose of this session is to ensure that all NCAs are familiar with the new features of the system, such as deduplication of PSURs and changes to the search screen which are provided in the new release of the PSUR repository. This session will be recorded and the recording will be made available via the eSubmission webpage.
- A dedicated Interactive Q&A session with MAHs on the use of PSUR repository v 01.04.00 is scheduled for the 28 October 09:00 – 10:30 (UK time). This session will be recorded and the recording will be made available via the eSubmission webpage. The purpose of this session is to ensure that all MAHs are familiar with the new features of the system, such as the grouping of associated submissions and changes to the product selection screen which are provided in the new release of the PSUR repository.
- The prioritisation of change requests for PSUR Repo v 01.05.00 is ongoing and the outcome of this exercise will shape the scope of this release which will include the following:
- API (automated 2-way exchange between NCA IT systems and PSUR Repository)
- Additional notification system (fulfilling post-audit requirement 1 and 3)
- Additional Change Requests (CRs) as prioritised by the Advisory Group
- There is an open call for interest for volunteers for the v 01.05.00 UAT which contains the API. Interested parties should contact turi.salami@ext.ema.europa.eu.
- MAHs are reminded that whenever a PSUR submission to the EMA is provided, even during the transition period leading up to the mandatory use of the PSUR Repository, such PSUR submission should be in eCTD format only for CAPs and in eCTD or Non eCTD electronic Submission (NeeS) format for NAPsthrough eSubmission Gateway/Web Client using XML delivery file created in the PSUR Repository user interface. In addition to this MAHs should note that as per the CMDh document “Requirements on Submissions for Periodic safety update reports (PSUR) to National Competent Authorities (NCAs) for products authorised via National Procedures, MRP and DCP (NAPs)” (CMDh/317/2014, Rev. 4, September 2015) “PSURs in unstructured PDF or paper format are not accepted for EU single assessment (PSUSA) and PSUR worksharing. If a PSUR for NAP(s) authorised in only one Member State is planned to be submitted in these formats for exceptional well justified reasons, the respective NCA should be contacted to confirm acceptability”, in line with the legislation requiring submission of PSUR electronically irrespective for CAPs or NAPs.
- Users should continue to report any issues they may have with the system through the PSUR Repository mailbox.
Week commencing 05 October 2015
- Release v.1.04.00 is scheduled for the 13 October 2015. The NCA and Industry user acceptance testing (UAT) finalised on the 02 October and the feedback is under assessment. The outcome will be presented in the next week’s bulletin.
- As part of this release the notifications will include an excel spreadsheet with the PSURs submitted to the repository which will facilitate the reconciliation process between the EMA and the Member States. This release also provides further usability improvements for Industry and a link between EURD and Art57.
- A dedicated MAH training on the new functionality from release v 01.04.00 of the PSUR repository is scheduled for the 14 October 09:00-10:30 (UK time). This session will be recorded and the recording will be made available via the eSubmission webpage.
- A dedicated NCA training on the new functionality from release v 01.04.00 of the PSUR repository is scheduled for the 15 October 09:00-10:30 (UK time). This session will be recorded and the recording will be made available via the eSubmission webpage.
- The prioritisation of change requests for PSUR Repo v 01.05.00 is ongoing and the outcome of this exercise will shape the scope of this release which will include the following:
- API (automated 2-way exchange between NCA IT systems and PSUR Repository)
- Additional notification system
- Additional Change Requests (CRs) as prioritised by the Advisory Group
There is an open call for interest for volunteers for the v 01.05.00 UAT which contains the API. Interested parties should contact turi.salami@ext.ema.europa.eu.
MAHs are reminded that whenever a PSUR submission to the EMA is provided, even during the transition period leading up to the mandatory use of the PSUR Repository, such PSUR submission should be in eCTD format only for CAPs and in eCTD or Non eCTD electronic Submission (NeeS) format for NAPsthrough eSubmission Gateway/Web Client using XML delivery file created in the PSUR Repository user interface. In addition to this MAHs should note that as per the CMDh document “Requirements on Submissions for Periodic safety update reports (PSUR) to National Competent Authorities (NCAs) for products authorised via National Procedures, MRP and DCP (NAPs)” (CMDh/317/2014, Rev. 4, September 2015) “PSURs in unstructured PDF or paper format are not accepted for EU single assessment (PSUSA) and PSUR worksharing. If a PSUR for NAP(s) authorised in only one Member State is planned to be submitted in these formats for exceptional well justified reasons, the respective NCA should be contacted to confirm acceptability”, in line with the legislation requiring submission of PSUR electronically irrespective for CAPs or NAPs.
Week commencing 07 September 2015
Week commencing 3 August 2015
- Release 1.03.00 of the PSUR Repository is made available for both MAHs and NCAs on 6 August delivering improved functionality and defect fixes which have been identified during the pilot phase. The updated system release notes and MAH and NCA user guides can be found under User documents on this page.
- The scope for release v.1.04.00 is being agreed with the advisory group and the release is planned for October 2015. The user acceptance testing (UAT) for the release is planned for September 2015. Call for interest for this UAT will be sent out early September.
Action items and reminders
- Training session for MAHs and other industry users concentrating on the new features of the PSUR repository v1.03.00 will be held on Wednesday 12 August 2015 at 1pm UK time. This session will be recorded and the recording made available on this page. Book your place by emailing PSURrepository@ema.europa.eu. It is recommended to review previous training material prior to attending this session as it will only cover the new functionality delivered in the new release.
- A follow-on interactive Q&A session on the use of PSUR Repository for MAHs and other industry users will be held on Thursday 10 September 2015 at 1pm UK time. This session will be recorded and the recording made available on the PSUR Repository webpage. Book your place by emailing PSURrepository@ema.europa.eu. It is strongly recommended to review available training material prior attending this interactive session where we will answer your questions on the new functionality. This Q&A session will not cover procedural aspects.
- Users should continue to report any issues they have with the system through the PSUR Repository mailbox PSURrepository@ema.europa.eu.
- List of available procedures for the August pilot on the basis of the submissions in the system has been distributed to the network. MAHs will be informed of the procedures taking part in the August pilot.
- On 11 June 2015 the EMA Management Board, based on a positive PRAC Recommendation and an independent audit report, announced that the PSUR repository meets the functional specifications as agreed in the ‘PSUR Repository functionalities to be audited’ document and concluded that it has achieved its full functionality. The legislation foresees that 12 months after the EMA Management Board announcement , the use of the repository for the submission, storage and retrieval of all PSURs and related documents (assessment reports) in the European Union will become mandatory (13 June 2016).
Full news item can be found on the EMA public website
- The scope for release v.1.03.00 has been agreed, and scheduled for early August 2015. The NCA and Industry user acceptance testing (UAT) will take place between the 17th and 23rd July. NCAs and industry representatives interested in participating in the UAT should contact Turi Salami (turi.salami@ext.ema.europa.eu) by the 25th June.
The main enhancements provided in this version are:
- Improvements to the product search functionality;
- Additional search criteria for PSURs and supplemental information within the non-EU Single assessment screen;
- Addition of national authorisation numbers to facilitate searches when uploading Non EU single assessment documents, and
- The addition of information regarding silent adoption/plenary discussion in the assessment report upload screen.
The “Kick-off” meeting with PSUR Repository Advisory Group to prepare the UAT will take place on the 26th June 2015.
Week commencing 29 June 2015
- Kick of meetings for the user acceptance testing (UAT) of release v.1.03.00 were held with industry and NCAs on the 26th and 29th June. The UAT will take place between the 17th and 23rd July with an introductory training session planned on the 17th July between 10:00-12:00 UK time. The aim of the training will be to present a demo of the new version, review the scripts for the UAT and present the feedback and support options for participants. Invitations to the UAT training session will follow shortly.
- The PSUR Repository team has initiated discussions with the network regarding the move from the pilot phase to the switch-on phase. Once the system moves to this phase the following will occur:
- Full ‘trial’ use of the PSUR Repository
- CAPs must use the repository
- EMA will manually upload any NAP submissions not submitted to the repository
The move to switch-on will allow the network to obtain the full benefits of the repository prior to the mandatory use.
Pre-conditions by the network for the switch-on will be presented for agreement in the July PRAC and CMDh meetings, together with a time schedule.
- The PSUR Repository team is currently preparing the list of available procedures for the July pilot on the basis of the submissions in the system. The same approach will be followed as was done in June, with the circulation of the list to the network and request for NCAs to express their interest in participating; MAHs will be informed thereafter.
- The PSUR Repository bulletin will remain on a bi-weekly schedule during the summer months.
Week commencing 08 June 2015
- As part of the PSUR Repository project the independent auditors PwC Luxembourg visited the EMA premises on the 2nd and 3rd of June, 2015 for a second round of fieldwork as follow-up to the initial audit held in February/March 2015. The purpose of this fieldwork was to verify if the findings identified during the initial audit had been tackled and the related recommendations implemented. The auditors were able to carry out their testing on recently deployed version 01.02.00 of the PSUR Repository, which became available on 01 June 2015. This release provides change requests enhancing the system and fixes defects since the start of the pilot phase. In particular, this release provides, one month earlier than planned, solutions for all remaining findings from the audit. The auditors concluded that the second run of tests demonstrates that all initial findings on the post-audit project plan have been fixed by EMA. The auditors did not identify any additional findings, concluding that the PSUR Repository is operational and allows a secure and smooth running of the business processes, thereby completely fulfilling the legal requirements. The independent audit report together with the PRAC Recommendation, issued in April 2015, will be presented at the EMA Management Board on 11 June 2015.
- The proposed scope for release v.1.03.00 is currently under review. The release is scheduled for the end of July.
Week commencing 06 April 2015
- During the April PRAC plenary the PRAC issued a positive Recommendation based on the independent audit report confirming that the PSUR repository meets the functional specifications as agreed in the ‘PSUR Repository functionalities to be audited’ document and concludes therefore that it has achieved its full functionality in the meaning of Art. 25a of Regulation (EC) No 726/2004.
- The PRAC Recommendation is a key milestone in the project; together with the independent audit report it forms the basis of the confirmation and announcement of the EMA Management Board that the repository meets the pre-agreed full functional specifications which is planned for June 2015.
Action items and reminders:
- DIA Information Day on New Services and Systems in Pharmacovigilance on 28 April 2015 at the EMA in London. 16:30 – 17:00 - SESSION 4: “PSUR REPOSITORY – Achievements and next steps”
Week commencing 16 March 2015
- The next version of the PSUR Repository (version 01.01) will be made available on 24 March 2015. This release provides change requests enhancing the system and fixes defects identified after the User Acceptance Testing (UAT) performed at the end of 2014 or since the start of the pilot phase.
- Marketing Authorisation Holders for Nationally Authorised products are reminded of their obligation to continue to submit PSURs in those Member States where the product(s) is authorised, in line with the standard submission requirements (please refer to Requirements for submissions for Periodic Safety Update Reports (PSUR) for MRP, DCP and National Products (NAPs). This requirement will remain until the use of the PSUR Repository is mandatory, which is planned for June 2016 at the earliest.
- A technical issue related to EMA eCTD review tool is affecting submissions of multiple eCTD NAP submissions. Guidance on these submissions is available here.
Week commencing 23 February 2015
- On the 24 February 2015 CAP MAH contacts and NAP QPPVs for procedures starting in May were invited to take part in the pilot. MAHs should confirm their participation in the pilot by providing the name of the product to PSURrepository@ema.europa.eu by the 2 March 2015.
- The PSUR Repository audit is ongoing.
Action items and reminders
- Pilot phase 2 planning: MAHs for procedures starting in May are requested to confirm participation to PSURRepository@ema.europa.eu by 02 March 2015.
- 3rd Industry Stakeholder Platform on the Operation of EU Pharmacovigilance Legislation with presentations on the PSUR Repository both from EMA and Industry - 13 March 2015.
- PSUR Repository interactive Q&A sessions for NCAs – 17 March 2015, 11:00 am UK time.
- PSUR Repository interactive Q&A sessions for MAHs - 05 and 19 March 2015, 09:00 am UK time.
Week commencing 09 February 2015
- Initial pilot phase started on 12 February with 9 CAP PSUSA procedures running through the repository. Standard business processes at NCAs and EMA will run in parallel to repository activity.
- Ten CAP PSUSA procedures starting in March have now been agreed to run in the repository.
- Trainings for new and existing gateway users occurred as planned on the 10 February and 12 February and can be found on the dedicated PSUR Repository webpage.
Week commencing 02 February 2015
- List of initial pilot procedures (CAPs only) to start in February has been agreed and will be presented during the February PRAC meeting.
- MAHs for March pilot procedure starts have been contacted to encourage and agree on use of the repository during the pilot phase.
- Planning of the extended pilot phase which will begin in May, and will include nationally authorised products, has commenced.
Action items and reminders
- PSUR Repository training for existing Gateway users - 10 February 2015
- PSUR Repository training for new Gateway users - 12 February 2015
Week commencing 26 January
2015
- Successful deployment of PSUR Repository into the live environment on 26 January 2015.
- Planning of phase I of the pilot phase where industry and NCAs may choose to use the PSUR Repository on a voluntary basis has commenced.
- Initial planning has begun on how to enhance the search and retrieval of non-EU single assessment PSURs (pure NAPs) in the repository.
|

