|
Previous eAF news
11-04-2024
Updated eAF v1.26.0.1 (Vet MAA) now available
An updated v1.26.0.1 Vet MAA eAF is now available on the eSubmission website.
A change has been implemented to fix a bug related to the navigation in the form after its finalisation.
It is recommended to use this latest form for new submissions. Please note that there is no
version number change and that the release notes will be updated and published in the relevant section of
the eAF page.
AND
electronic Application Forms (eAFs) not available for use between 11th and 16th April
2024 due to planned maintenance
Due to planned maintenance activities, the interactive PDF eAFs (human and
veterinary variation and MAA forms and the renewal form) and the PLM Portal web-based eAF
will be unavailable for use between 11th and 16th April 2024.
Please refer to the news published on 15 March 2024 for the complete details of the planned
maintenance and we remind you that we strongly recommend to use the interactive pdf eAF instead of
the web-based eAF for submissions at least until 26 April 2024 to prevent validation issues
and potential delays.
15-03-2024
electronic Application Forms (eAFs) not available for use between 11th and 16th April
2024 due to planned maintenance
Due to planned maintenance activities, the interactive PDF eAFs (human and
veterinary variation and MAA forms and the renewal form) and the PLM Portal web-based eAF
will be unavailable for use between 11th and 16th April 2024.
Additionally, as announced in December 2023, the PLM Portal web-based eAF will
be affected by the data load of Centrally Authorised Products (CAPs) and Nationally Authorised Products
(NAPs) into Product Management Service (PMS) as well as the simultaneous load of updated* CAPs to
web-based eAF.
This load of data into PMS is a necessary step in preparation to the forthcoming launch of the Product
User Interface view and later edit functions as well as making NAPs data available for the PLM Portal
web-based eAF.
The data load will take place from 11 to 16 April 2024.
During this timeframe, the interactive PDF eAF and the PLM Portal web-based eAF will experience a
downtime.
Please note that, during the preparation for the updated* CAPs load in the PLM Portal web-based
eAF, the match-merge** operation will result in:
- changes to product names;
- introduction of new medicinal products through splitting**;
- potential alterations to packages.
These changes will impact how products appear in application forms. Therefore, we
strongly recommend, with immediate effect, that no applications that might
require any update of the eAF, during or after the product upload, are submitted to EMA
using the PLM Portal web-based eAF. We strongly recommend to use the interactive
pdf eAF instead of the web-based eAF for submissions at least until 26 April 2024 to prevent
validation issues and potential delays.
Please note that if you have already submitted a web-based eAF, or it is expected that there will be no
need to update the form and/or the procedure will conclude prior to the downtime, you can continue using
the web-based eAF.
Please note that, except for the downtime period, the web-based eAF is expected to remain accessible
to applicants to familiarise themselves with changes to the products, and for training purposes.
*Including split & match-merge processes. Please refer to EU IG (Implementation Guide) Chapter 7 for a detailed description of these steps.
**The 'Match-merge' process serves to include data from XEVMPD to products already released in PLM Portal.
The 'split' process serves to make released products ISO-IDMP compliant. Both processes are explained in
detail in EU IG Chapter 7
29-02-2024
Updated eAF v1.26.0.1 (Vet MAA) now available
An updated v1.26.0.1 Vet MAA eAF is available starting with 29 February 2024, 18:00 CET.
A change has been implemented to add "United Kingdom (Northern Ireland)"" in the Member state selection in sections 2.4.1 and 2.4.4.
It is recommended to use this latest form for new submissions. Please note that there is no version number change and that the release notes will be updated and published in the relevant section of the eAF page.
01-02-2024
User Experience of the current PDF eAFs completion on the PLM Portal
The electronic Application Form (eAF) Product Team would greatly appreciate to receive your feedback on your current experience in terms of time spent in filling in the interactive pdf electronic Application Form (eAF) for Variations for Centrally Authorised Products (CAPs) and Nationally Authorised Products (NAPs), and Initial Marketing Authorisation.
It is acknowledged that different types of procedures (e.g. Type IA, Type II applications, worksharing, and groupings) may require varying time commitments. We would therefore appreciate if, in your answer, you could provide the average time spent and resources used to fill in the forms.
Your feedback will provide us an indication on the current performance requirements and will be our starting point for improving future user experience.
You can find the questionnaire to fill in at the following link:
https://ec.europa.eu/eusurvey/runner/Eusurvey_PLMPortal_UX_eAFcompletion
Please note that this survey should not take more than 5 minutes to complete, and the responses will remain anonymous.
We kindly ask you to respond to the survey by Thursday 29 February 2024.
Please send any question to plm.valuestream@ema.europa.eu or post them via the PLM Portal Forum.
07-12-2023
Updated eAF v1.26.0.0 (Human MAA) and v1.26.0.1 (Vet MAA) now available
Updated v1.26.0.0 Human MAA eAF is now available on the eAF website. The form is ready for immediate use. The change implemented in this version is a minor bug fix (related to Annex 5.19).
It is strongly recommended to use this latest version (document properties date 27.10.2023). Please note that there is no version number change and that the release notes are published in the relevant section of the eAF webpage.
Updated v1.26.0.1 Vet MAA eAF is now available for immediate use.
The changes in this version relate to label and text changes only. Additionally, the version number has updated from v1.26.0.0 to v1.26.0.1.
The version 1.26.0.1 can be used immediately, and it is strongly recommended that it will be used for all new applications as soon as possible, however, the one-month transitional period will run until 8th of January 2024 after which the use of version 1.26.0.1 for the Veterinary MAA form will become mandatory.
The version 1.26.0.0 has now been removed from the eAF website however users can continue to submit applications using this version until 7th of January 2024.
Please note that as there are no technical changes, it is possible to import the xml from version 1.26.0.0 into version 1.26.0.1 to avoid any need for manual effort.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure, please note that if you need to provide an updated form for a procedure that has started prior to 8th of January, you should use the previous version.
Updated release notes are published in the relevant sections of the eAF webpage.
30-11-2023
Updated version of the eAF v1.26.0.0 (variation and renewal)
An updated version 1.26.0.0 of the Variation and Renewal (both Human and Veterinary) eAF is available starting with 28 November 2023, 18:00 CET.
A change has been implemented to allow Non-Current terms to be selected in the "Pharmaceutiacal form" lists.
It is recommended to use this latest form for new submissions. Please note that there is no version number change and that the release notes were updated and published in the relevant section of the eAF page.
04-10-2023
Updated user guide for the eAF for MAA available on the CMDh website
A new version of the joint EMA/CMDh User guide for the electronic application form for a Marketing Authorisation is available on the CMDh website.
Human variations form updated timeline
Please find the September 2022 version of the DADI Human Variations Forms
timeline, outlining the updated DADI BETA UAT timeframe. The UAT has started on 19th September and will end on the 30th September 2022.
31-07-2023
Updated version of the eAF v1.26.0.0 (veterinary variation)
An updated version 1.26.0.0 of the veterinary variation eAFs is available starting with 24 July 2023.
A single change has been implemented to address a non-blocking bug (related to the formatting of the page).
It is recommended to use this latest form for new submissions (document properties date 24.07.2023). Please note that there is no version number change and that the release notes are not published for this minor change at this time.
28-06-2023
Updated regulatory practical guidance
The European Medicines Agency practical guidance on the application form for centralised type IA and IB variations (europa.eu) has been updated and can also be found under the section "Regulatory".
15-06-2023
Planned maintenance of the eSubmission systems on 17 June 2023
Due to planned maintenance, the eSubmission systems (including eAF and PLM Portal eAF) will not be available on Saturday 17 June 2023, between 8:00 and 16:00. For any further information, please contact EMA Service Desk.
And
Updated version of the eAF v1.26.0.0 (human variation)
An updated version 1.26.0.0 of the human variation eAFs is available starting with 14 June 2023.
A single change has been implemented to address a non-blocking regression bug (related to the declaration on the manufacturers and MAH).
It is recommended to use this latest form for new submissions (document properties date 14.06.2023). Please note that there is no version number change and that the release notes are not published for this minor change at this time.
30-05-2023
Updated version of the eAF v1.26.0.0 (human variation)
An updated version 1.26.0.0 of the human variation eAFs will be available starting with 31 May 2023, 20:00.
A single change has been implemented to address a non-blocking bug. There is a very limited impact to users of the forms. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human).
It is recommended to use this latest form for new submissions (document properties date 25.05.2023), however you can finalise the existing forms (if any) in the previous version. Please note that there is no version number change and that the release notes are not published for this minor change at this time.
10-05-2023
eAF PDF working as expected
The selection of active substances is now possible and eAF PDF is working as expected. Should you encounter any issues, please raise a ticket in EMA Service Desk (https://support.ema.europa.eu/esc).
08-05-2023
eAF PDF not working as expected
Please note the eAF PDF is not working as expected (the active substances cannot be selected from the database). Our colleagues are working on fixing the issue and once the eAF PDF will be fully functional, a message will be posted on this page and the users who have raised a related ticket in EMA Service Desk will be contacted as soon as possible.
17-03-2023
Planned maintenance of the eSubmission systems on Saturday 25 March 2023
Planned maintenance of the eSubmission systems on Saturday 25 March 2023
The eSubmission systems are unavailable for planned maintenance and will be unavailable from Saturday 25th of March 2023 08:00 until 18:00 due to essential maintenance. For any further information, please contact Service Desk
14-10-2022
Updated version of the eAF v1.26.0.0 (human variation) now available
An updated version 1.26.0.0 of the human variation eAFs is now available.
A single change has been implemented to emphasize the mandatory use of OMS for centralised procedure by updating the Declaration label in the Proposed section. There is a very limited impact to users of the forms. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human).
Please note that an updated EMA practical guidance on the eAF for Type IA and IB variations is now available and this updated version of the guide addresses the use of this important declaration.
It is strongly recommended to use this latest version (document properties date 3.10.2022).
Please note that there is no version number change.
Please note that the release notes are not published for this minor change at this time.
14-06-2022
Updated versions of the eAF v1.26.0.0 (bug fix version) now available
Updated versions 1.26.0.0 of the human and veterinary variation eAFs are now available.
The changes implemented in this version are business rule corrections only, relating to mandatory use of OMS for centralised procedure and business rule in section 4b Paediatrics in the human variation form.
There is a very limited impact to users of the forms. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human) and 28th January 2022 (veterinary).
It is strongly recommended to use this latest version (document properties date 30.05.2022).
Please note that there is no version number change.
10-06-2022
Updated versions of the eAF v1.26.0.0 (bug fix version) go-live on 14th June 2022
Updated versions 1.26.0.0 of the human and veterinary variation eAFs will be released on the eAF website on Tuesday 14th June 2022.
The changes implemented in this version are business rule corrections only, relating to mandatory use of OMS for centralised procedure and business rule in section 4b Paediatrics in the human variation form. There is a very limited impact to users of the forms. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human) and 28th January 2022 (veterinary).
It is strongly recommended to use this latest version (document properties date 30.05.2022). Please note that there is no version number change.
26-04-2022
Updated versions of the eAF v1.26.0.0 (bug fix version) now available
An updated human and veterinary MAA forms, Variation forms and the updated Renewal form are ready for immediate use.
The changes implemented in this version are bug fixes and business rule corrections only and there is a limited impact to users. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human) and 28th January 2022 (veterinary).
It is strongly recommended to use this latest version (document properties date 26.04.2022).
Please note that there is no version number change.
31-03-2022
Updated versions of the eAF v1.26.0.0 (bug fix version) now available
Updated versions 1.26.0.0 eAFs are now available. An updated human and veterinary MAA forms, Renewal form and veterinary variation form are ready for immediate use. The changes implemented in this version are minor bug fixes only and they do not affect most users. The use of the v1.26.0.0 is mandatory since 1st January 2022 (human) and 28th January 2022 (veterinary) and the users can select if they wish to continue using the previously published version or the updated version (there is no version number change or any changes to the Data Exchange Standard (DES)).
More details can be found from the release notes. Please note that there are no updated release notes for the veterinary variation form, however, there has been a very minor change to fix the UPD Product Identifier field length to allow inclusion of the UUID.
11-02-2022
Technical issue on the eAF webpage affecting availability of the latest versions of the eAF v1.26.0.0 has been fixed
The updated versions 1.26.0.0 eAFs (Veterinary MAA and variation, human variation) were published on the eAF website on 20th of January 2022.
A technical issue has been identified which means that these latest versions were replaced by earlier versions (at some point around 5th of February). The issue has been now fixed and the latest version of the forms are now again available, but please note that if you have downloaded/used an eAF from the eAF website recently, please check the document properties to ensure that you are using the correct, latest version dated 17/01/2022.
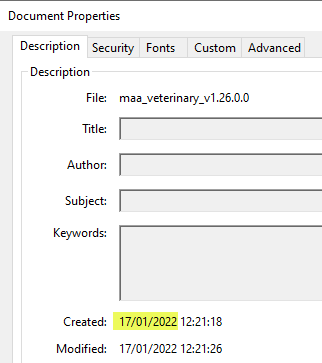
28-01-2022
Mandatory use of eAF v1.26.0.0 for all new veterinary applications
The use of the updated version of 1.26.0.0 eAFs for all new veterinary applications is now mandatory.
Please note that there is no transitional period and the v1.25.0.0 cannot be used for any new procedures submitted on or after 28th January 2022.
The version 1.25.0.0 has now been removed from the website, however, it should be noted that the version of the form should not be changed during an ongoing procedure.
This also applies to the veterinary procedures which have started prior to 28th of January 2022.
20-01-2022
Updated versions of the eAF v1.26.0.0 now available
Updated versions 1.26.0.0 eAFs are now available on the eAF website. An updated human variation form is ready for immediate use. The change implemented in this version is a minor business rule change only and it will not affect most users. The use of the v1.26.0.0 is mandatory since 1st January 2022 and the users can select if they wish to continue using the previously published version or the updated version.
Updated forms for veterinary applications are available for applicants and MAHs to familiarise themselves with the updated forms prior to the mandatory use of this version for veterinary submissions from 28th of January 2022. Please note that there will be no transitional period once these forms go-live on the 28th of January. The v1.25.0.0 cannot be used for any new procedures starting after 28th January 2022.
The main changes in this updated version of the veterinary forms (maa and variation) relate to the implementation of the Regulation (EU) 2019/6 for Veterinary Medicinal Products and the changes are those that were previously indicated as known issues in the release notes and other issues that were raised during the UAT and other issues that have been discovered by users since the publication of the forms on the 1st of December 2021.
The version 1.26.0.0 cannot be used for procedures prior to 28th January 2022, however, in the view of significant changes in the forms it is strongly recommended that applicants will carefully review the updated forms (maa and variation) prior to the mandatory use deadline in order to identify and report any issues in the implementation and thus allowing an opportunity to fix any issues found prior to 28th January 2022.
The version 1.25.0.0 will remain available for use for veterinary procedures (maa and variation) until the mandatory use of v1.26.0.0
Applicants are reminded that the version of the form should not be changed during an ongoing procedure. This also applies to the veterinary procedures which have started prior to 28th of January 2022, it is important to note that the version of the form must not be changed during an ongoing procedure.
01-12-2021
eAF v1.26.0.0 now available
New version 1.26.0.0 of all eAFs is now available on the eAF website. The forms for human applications (maa, variation and renewal) are ready for immediate use. The use of the v1.26.0.0 for human procedures becomes mandatory after a short one-month transitional period on 1st January 2022. The version v1.25.0.0 of the human forms has been removed from the eAF website, however users can continue to submit new applications using this version until the end of December 2021.
The main change in this version of the forms for human use relate to the mandatory use of OMS for Centralised Procedure applications. This version of the forms removes the free text fields in the forms when EU authorisation/Centralised Procedure is selected. Additionally, a bug fix relating to Medical Device section in the variation form has been provided.
The forms for veterinary applications are available for applicants and MAHs to familiarise with the updated forms prior to the mandatory use of this version for veterinary submissions from 28th of January 2022. Please note that there will be no transitional period once these forms go-live on the 28th of January. The v1.25.0.0 cannot be used for any new procedures starting after 28th January 2022.
The main changes in this version of the veterinary forms (maa and variation) relate to the implementation of the Regulation (EU) 2019/6 for Veterinary Medicinal Products.
The version 1.25.0.0 will remain available for use for veterinary procedures (maa and variation) until the mandatory use of v1.26.0.0
Applicants are reminded that the version of the form should not be changed during an ongoing procedure. This also applies to the veterinary procedures which have started prior to 28th of January 2022, it is important to note that the version of the form must not be changed during an ongoing procedure.
26-10-2021
Updated draft technical documents for eAF v1.26.0.0 now available
Updated draft DES summary and draft XSD files for the Vet MAA and variation forms are now available.
And
Updated renewal form and veterinary maa eAF v1.25.0.0 now available
Updated versions of the renewal form and the vet maa form v1.25.0.0 are now available on the eAF website. There is no change in the version number. This is a bugfix update to fix 2 minor technical bugs which do not affect all users.
25-10-2021
Call for volunteers for testing eAF v1.26.0.0 veterinary variation and maa forms (reflecting the VMP-Reg)
The version 1.26.0.0 of the electronic Application Forms (eAFs) is planned for release on 1st December 2021 (for information only) and mandatory use from 28th of January 2022. The release v1.26.0.0 will provide a major change in both variation and maa forms for veterinary applications to align with the new Veterinary Regulation (VMP-Reg). These changes will be detailed in the release notes. User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
The testing by Industry and NCAs is planned to take place as follows:
- Industry: from Fri 29/10/21 to Tue 09/11/21
- NCAs*: from Wed 03/11/21 to Fri 12/11/21 (*Based on the eAFs received from Industry)
Please note that these dates are subject to change depending on the outcome of internal UAT. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu .
13-10-2021
Updated versions of eAF v1.25.0.0 now available - bug fixes
Updated bug fix versions 1.25.0.0 of all four eAFs are now available for immediate use. The variation and renewal form proof of payment section has been fixed and in the Human and Veterinary MAA forms the OMS selection for the admin address in 2.5.1.a has now been fixed. More details can be found from the summary release notes.
If you have already started to fill in the form in a version where these issues are occurring, you can export the xml and import it into the newly published version. This should fix the issue.
11-10-2021
Questions and Answers document on the Mandatory use of OMS for CAPs now available
An updated version of the Questions and Answers document to support applicants and Marketing Authorisation holders in the implementation of the mandatory use of OMS for CAPs can be found here.
Please note that a training webinar on OMS will be held on 21st of October 2021, where registered participants will have the opportunity to clarify any outstanding questions. The webinar will be recorded and made available after the event.
06-10-2021
Questions and Answers document on the Mandatory use of OMS for CAPs now available
The use of Organisational Maintenance Services (OMS) will become mandatory for Centrally Authorised Products (CAPs) from 1st of November 2021. A Questions and Answers document to support applicants and Marketing Authorisation holders is now available.
01-10-2021
eAF v1.25.0.0 now available
New version 1.25.0.0 of all four eAFs is now available for immediate use.
The main changes in this version of the forms relate to the implementation of the Medical Device Regulation Art 2(1) of Regulation (EU) 2017/745 and include other changes as implemented in the latest version of the NTA application forms.
The changes, in summary, are an updated section on Medical Devices in the human MAA form, addition of a new section on Medical Devices in the variation form, addition of new sections on parallel variations and on Harmonisation relating to National Variations. An incorrect business rule relating in the Renewal form for excipients has also been fixed in this version.
The version 1.25.0.0 can be used immediately, and it strongly recommended that it will be used for applications for products containing medical devices as soon as possible, however, the one-month transitional period will run until end of October after which the use of version 1.25.0.0 of the forms will become mandatory.
The version 1.24.0.1 has now been removed from the eAF website however users can continue to submit applications using this version until the end of October 2021.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
And
Mandatory use of OMS in eAFs from 1st of November 2021
The use of OMS in the eAFs will become mandatory from 1st of November 2021. Please note that a release to remove the free text fields in the human forms for Centralised Procedure (v1.26.0.0) is planned for release in early December 2021.
Version 1.26.0.0 will be mandatory for use for human domain from 1st of January 2022.
Please note that this version cannot be used for veterinary submissions prior to 28th January 2022. For veterinary domain, there will be no transitional period and the version 1.26.0.0 will become mandatory on the 28th January 2022. The previous version of the forms should be used for already ongoing procedures and applicants are reminded that the version of the form should not be changed during an ongoing procedure.
28-09-2021
Further extension to deadline for mandatory use of OMS for all CAP submissions
In order to allow applicants and MAHs to timely register in OMS, the deadline for mandatory use has been further extended and the mandatory use of OMS Organisation Management Service (OMS) for CAPs now starts on 1st of November 2021. Early registration of site(s)/organisations in OMS is encouraged.
The EMA would like to emphasise the importance of site registration in OMS before the regulatory submission. This will avoid any delay in the start of these applications as applicants will be requested to register the site(s)/organisations during validation and prior to the start of the procedure.
A Questions and Answers document providing further information and details will be published shortly.
10-08-2021
DADI Project update now available
DADI project update is now available here. This update includes a timeline for the first forms to be released, a list of features of the human variation form and an updated questions & answers document.
02-08-2021
Call for volunteers for eAF v1.25.0.0 (reflecting the updated Medical Devices regulation)
The version 1.25.0.0 of the electronic Application Forms (eAFs) is planned for release at the end of September 2021 with short 1-month transitional period and mandatory use from 1st of November 2021. The release v1.25.0.0 will provide a major change into the section 2.2.4 (Medical Device) of the human MAA form as well as adding similar section in the human variation form. Additionally, there are other changes across all forms. These changes will be detailed in the release notes and in a release specific presentation. User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
The testing by Industry and NCAs is planned to take place as follows:
- Industry: from Tue 24/08/21 to Wed 01/09/21
- NCAs*: from Mon 30/08/21 to Fri 03/09/21 (*Based on the eAFs received from Industry)
Please note that these dates are subject to change depending on the outcome of internal UAT. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu.
Further details on the release scope and the confirmed dates for the UAT will be provided soon.
22-07-2021
Extended deadline for mandatory use of OMS for all CAP submissions
The EMA would like to inform applicants and Marketing Authorisation Holders (MAHs) of human and veterinary medicinal products that as part of the development of the Substance, Product, Organisation and Referential (SPOR) data management services, and in anticipation to the upcoming Digital Application Dataset Integration (DADI) project, the registration in Organisation Management Service (OMS) will become mandatory for new sites and organisations to be registered for a Medicinal Product as part of any regulatory procedure submitted to the Agency. This includes but is not limited to pre-submission phase activities (eligibility requests, pre-submission meeting requests, change in contact person requests, ...), marketing authorisation applications, line extensions and variations (type IA, IB and II) and renewals.
In order to allow applicants and MAHs to timely register in OMS, the previously communicated deadline of 1 August 2021 is extended until the end of September 2021. Early registration of site(s)/organisations in OMS is encouraged.
In the future, registering the site(s)/organisations in OMS will also become mandatory for national procedures. The Agency will provide additional information in due time.
The EMA would like to emphasise the importance of site registration in OMS before the regulatory submission. This will avoid any delay in the start of these applications as applicants will be requested to register the site(s)/organisations during validation and prior to the start of the procedure.
12-07-2021
Mandatory use of OMS in eAF for all CAP submissions
The EMA would like to inform applicants and Marketing Authorisation Holders (MAHs) that as part of the development of the Substance, Product, Organisation and Referential (SPOR) data management services, and in anticipation to the upcoming Digital Application Dataset Integration (DADI) project replacing the PDF format eAFs, the registration in Organisation Management Service (OMS) will become mandatory from the 1st of August 2021 for new sites and organisations to be registered for a Medicinal Product as part of any regulatory procedure submitted to the Agency. This includes but is not limited to pre-submission phase activities (eligibility requests, pre-submission meeting requests, change in contact person requests, ...), marketing authorisation applications, line extensions and variations (type IA, IB and II) and renewals.
The EMA would like to emphasise the importance of site registration in OMS before regulatory submissions. This will avoid any delay in the start of these applications as applicants will be requested to register the site(s)/organisations during validation and prior to the start of the procedure.
25-03-2020
Digital Application Dataset Integration (DADI) project
The European Medicines Regulatory Network has launched a new telematics project called the Digital Application Dataset Integration project (DADI) to modernise and improve use of the EU electronic Application Forms (eAFs). DADI has been established as the successor to the Common European Single Submission Portal (CESSP) phase 1 project.
More details are available here.
15-12-2020
eAF v1.24.0.1 now available
New version 1.24.0.1 of all four eAFs is now available to allow the users a short transitional period before the start of the mandatory use of the form. The changes in this version of the forms (v1.24.0.1) relate to the Member State, OMS and Country fields in all 4 forms and the changes are implemented due to the end of the transitional period following Brexit and in the view of the Northern Ireland protocol.
The changes, in summary, are either addition of a new ‘country’ United Kingdom (Northern Ireland) in the EU and EEA country lists and removal of United Kingdom from the EU and EEA country lists in RMS. Additionally, 3 new country ‘groupings’ have been created in RMS to either remove or add United Kingdom or United Kingdom (Northern Ireland) where these terms should or shouldn’t be displayed. Some minor amendments to which country grouping certain fields are pointing to have also been made.
The short transitional period will coincide with the transitional period with the previous version of the eAFs (v1.23.1.3 variation, renewal, MAA Vet)/1.23.1.4 MAA Human) which was initially planned to end on 15th of December 2020.
The users are now allowed to submit applications using version 1.23.1.3/1.23.1.4 until the end of 2020. The version 1.23.1.3/1.23.1.4 has been removed from the eAF website however, it does remain acceptable for submissions until the end of 2020.
The new Brexit related version can be used for submissions for procedures starting after 1.1.2021.
There will be a short transitional period for the use of previous version 1.24.0.0 until the 15th of January for those applications that do not require use of terms United Kingdom or United Kingdom (Northern Ireland).
From 1st of January 2021 the term United Kingdom will be removed from EU/EEA country grouping and the term will no longer be available for selection in any version of the forms (in the fields where EU/EEA countries are listed).
This version also includes a change in the Variation form, form validation rules for National Authorisation applications for grouping of a single variation scopes for multiple products.
More details can be found from the release notes and a presentation on changes below.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
02-12-2020
eAF issues with webservices fixed
The issue affecting the ‘Substance Type’ field in both, human and veterinary MAA forms has now been fixed and all the previously available substance types are now again available.
For any issues with the eAFs, please raise a call via the EMA service desk.
01-12-2020
eAF issues with webservices - Update
An issue relating to the eAF webservice update which affected availability of terms in the ‘Pharmaceutical Form’ field in all 4 forms has been fixed (Monday 30th November). All pharmaceutical forms should now be again in the eAFs. Please note that as this issue affected the webservices to the form, not the forms themselves so there is no need to change the form, just ‘update lists’ if the terms are not yet visible.
Additionally, another issue affecting the ‘Substance Type’ field in both, human and veterinary MAA forms has been detected and we are working on a further fix to solve this issue as soon as possible.
For any issues with the eAFs, please raise a call via the EMA service desk.
26-11-2020
eAF issue with webservices
An issue relating to the eAF webservice update has affected availability of terms in the ‘Pharmaceutical Form’ field in all 4 forms. We have released a fix that has partially solved the issue; however, we are aware that there are still many combined pharmaceutical forms that are currently unavailable in the eAFs. Please note that as this issue affects the webservices to the form, not the form itself, also previous versions of the form are affected.
We are working on a further fix to solve this issue as soon as possible.
For any issues with the eAFs, please raise a call via the EMA service desk.
27-10-2020
eAF v1.24.0.0 important note to users
An updated version of the variation eAF v1.24.0.0 is now available. This release which does not change the form version number, provides a fix to the Adobe backwards compatibility issue and it should solve the issues some users have experienced with the scope selection dropdown list in section 3. This version also includes a change in the form validation rules for grouping of Type IA or Type IAIN variations.
Please ensure that upon opening the eAF you ‘trust’ the form by clicking the small exclamation mark at the top of the left-hand adobe pane and then selecting the trust option from the yellow banner which will open across the top of the forms. The drop-down menu’s will not work if the form has not been trusted.

The Presentations for applicants and NCAs on the release v1.24.0.0 have been updated and are available below.
For any issues with the eAFs, for example reporting missing scopes, please raise a call via the EMA service desk.
08-10-2020
eAF v1.24.0.0 important note to users
The variation form can only be used with Adobe Reader version DC. The functionalities in variation selection drop down list in section 3 will not work with older versions (e.g. reader 2017, X or XI).
Please ensure that if you have Adobe Acrobat or Acrobat Pro 2017, X or XI installed, you close all documents that have been opened using Acrobat/Acrobat Pro or documents that contain Adobe sign signature before opening the eAF using reader DC as these may prevent the drop down list of scopes in section 3 working as expected.
Please note that we are working hard to find a solution to this cross-interference issue as soon as possible.
Please ensure that upon opening the eAF you ‘trust’ the form by clicking the small exclamation mark at the top of the left-hand adobe pane and then selecting the trust option from the yellow banner which will open across the top of the forms. The drop-down menu’s will not work if the form has not been trusted.
The presentation on the release v1.24.0.0 has been updated and is available below.
For any issues with the eAFs, for example reporting missing scopes, please raise a call via the EMA service desk.
15-09-2020
Version 1.24.0.0 of all four electronic Application Forms (eAFs) is now available. The release v1.24.0.0 provides a major change in section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation). Some defects have been fixed in MAA human, MAA vet and renewal (H&V forms.
The new version is implemented with 3 months transitional period. Mandatory use of v1.24.0.0 will start on 16th December 2020.
More details can be found in the release notes and the updated Practical user guide for electronic Application Forms as well as the presentation on changes.
Please note that due to the major change in the variation form, data imports from older versions of the variation form will not work.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
11-09-2020
Version 1.24.0.0 of all four electronic Application Forms (eAFs) will be made available on 15th September 2020 with a slightly longer, 3 months transitional period and mandatory use from December 2020. The release v1.24.0.0 will provide a major change in section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation). There has been minor defect fixes in the other 3 forms (MAA human, MAA vet and renewal (H&V).
20-07-2020
An updated release milestone plan and release schedule for the electronic Application Forms (eAF) is available below.
Version 1.24.0.0 of all the 4 electronic Application Forms (eAFs) is planned for release in September 2020 with a 2 month transitional period, and mandatory use from November 2020.
The release v1.24.0.0 will provide a major change into the section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation).
Please note that both MAA forms, human and veterinary, have been added into this release following a discovery of a defect in the forms.
The User Acceptance Testing (UAT) to support the release of this next version of the forms started on Friday 17th of July 2020.
Due to the delay in starting testing, we are now testing in parallel with both user groups (Industry and NCAs) and the UAT will run until Tuesday 4th of August 2020.
If you have not yet registered, however, wish to participate in the UAT, please contact us by email to eSubprogofficer@ema.europa.eu
29-06-2020
There is a short delay in the start of the UAT for the eAF v1.24.0.0 – new dates will be communicated very soon. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu
11-06-2020
eAF v1.24.0.0 release milestone plan and updated release schedule for eAF now available
An updated release milestone plan and release schedule for the electronic Application Forms (eAF) are now available below.
Version 1.24.0.0 of the variation and renewal electronic Application Forms (eAFs) is planned for release in September 2020 with a 2 months transitional period and mandatory use from November 2020. The release v1.24.0.0 will provide a major change into the section 3 of the variation form by further integrating with RMS from SPOR (scopes and conditions and documentation). User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
The testing by Industry and NCAs is planned to take place as follows:
- Industry: from Mon 29/06/20 to Fri 03/07/20
- NCAs*: from Mon 06/07/20 to Fri 10/07/20 (*Based on the eAFs received from Industry)
Please note that these dates are subject to change depending on the outcome of internal UAT. If you wish to participate in the UAT, please register by email with eSubprogofficer@ema.europa.eu
Further details on the release scope and the confirmed dates for the UAT will be provided soon.
31-03-2020
In the view that many applicants and MAHs are now working from home due to the Covid-19 situation, we would like to remind the users of the eAF functionality which may facilitate working during exceptional circumstances with variable internet bandwidths.
The electronic Application Forms can be saved directly from the eSubmission website to the users local machine and most fields can be filled in off-line, without access to internet. Only those fields that require substance/term related searches, and the use of OMS for address details requires connection to the internet. Validation feature and most dropdown lists are loaded to the form at the time when the form is opened and can be used even when the user is not connected to the internet enabling most of the form to be filled in without internet connection.
The performance of the forms with regards to the speed is unfortunately not optimal, mainly due to the technology used and the multiple business rules built in to the form, and hence, the network is working hard on the replacement of the PDF format forms with a web-based forms. The new web based forms for human and veterinary MAAs are planned for release later this year (timelines subject to change due to Covid-19 situation).
The eAFs are submitted as a part of the dossier which, for centralised procedure applications, must be submitted via the eSubmissions Gateway. In case the users are experiencing issues with the eAFs or preparing and sending submissions via the eSubmission Gateway or Web Client, we strongly recommend raising a ticket with detailed description of the issue via the EMA service desk portal.
It is important to include as many details as possible of the issue and, for example attaching screenshots or the form in question to aid investigation for the reasons of the issue.
04-06-2020
Computer application maintenance – Sunday 7 June 2020
Essential maintenance work will be carried to eSubmission applications as part of EMA’s computer application maintenance work this weekend. As a result, eSubmission applications will be intermittently unavailable to users between 13:00 – 17:00hrs on Sunday, 7 June 2020.
24-03-2020
Computer application maintenance weekend 28-29 March 2020
Essential maintenance work will be carried out to all Telematics applications as part of EMA’s computer application maintenance work during the last weekend in March. As a result, eAF will be unavailable to users between 18:30hrs on Friday, 27th March and 08:00hrs on Monday, 30th March 2020
09-12-2019
Version 1.23.1.4 of the Human Marketing authorisation Application (maa) electronic Application Form (eAF) is now available. The release v1.23.1.4 is a bug fix release to correct business rules implementation in sections 1.4 and 1.5. Further details are available on the release notes. This bug fix release does not affect the Data Exchange Standard (DES). Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
10-10-2019
Version 1.23.1.3 of the 4 electronic Application Forms (eAF) is now available. This release (v1.23.1.3) is a bug fix release to correct a number of issues which affect the forms. Further details are available in the release notes. This bug fix release does not affect the Data Exchange Standard (DES).
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
01-10-2019
Version 1.23.1.3 of the 4 electronic Application Forms (eAF) will be available 10 October 2019. The release v1.23.1.3 is a bug fix release to correct a number of issues affecting the forms. More details on the fixes will be available in the release notes which will be published together with the updated forms. This hotfix release does not affect the Data Exchange Standard (DES).
Applicants are reminded that the version of the form should not be changed during an ongoing procedure.
22-07-2019
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services on Tuesday, 23 July 2019. The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology will continue to work during this maintenance activity.
19-07-2019
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services between 18:00hrs (CEST) and 19:00hrs (CEST) on Friday, 19 July 2019 and between 21:00hrs (CEST) and 22:00hrs (CEST) on Sunday 21 July 2019. The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology will continue to work during this maintenance activity.
26-06-2019
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services on Saturday, 29 June and Sunday, 30 June 2019. The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas).
There is no impact to the Variation application form.
All other dropdown menus for controlled terminology will continue to work during this maintenance activity.
03-06-2019
A webinar providing an update of the CESSP phase 1 project (converting the current eAFs MAA human and veterinary into web based forms) will be held on 5 June 2019. This webinar is aimed for Regulators and instructions on how to connect have been sent out by email to representatives of National Competent Authorities.
23-05-2019
The presentation and recording from the webinar providing an update to Industry about the CESSP phase 1 project held on 7th of May 2019 is now available here.
15-04-2019
A webinar providing an update of the CESSP phase 1 project (converting the current eAFs into web based forms) will be held on 7 May 2019 from 10:00 to 11:30 CET. This webinar is aimed for applicants (from pharmaceutical industry). A separate session aimed for regulators will be be organised in near future.
Please note that participation in this webinar is on first come first serve basis as the maximum capacity of the virtual meeting room is limited to 200 participants. If multiple users from your organisation are attending, please share the connection with your colleagues.
The webinar will be recorded and the recording will be made available on the CESSP webpage after the webinar.
How to login is availble here.
10-04-2019
The applicants are reminded that eAFs should be edited and signed using Adobe Reader. Using Adobe Acrobat Pro may lead to issues when the regulators review the applications using Adobe Reader and this may lead to validation issues and delays in processing the applications. More information can be found in the eAF Q&A document.
28-03-2019
The release notes for MAA Human and Veterinary electronic Application Forms (eAF) and the Renewal eAF have been updated to reflect new known issues.
26-03-2019
eAF dropdown lists not working?
Users are reminded that the eAFs will need to be ‘trusted’ in order for the dropdown lists to work. To ‘trust’ the forms, please click the exclamation mark on the top of the left hand pane and select ‘options’ to open the trust options. Select ‘trust this document always’ to proceed.

15-03-2019
Version 1.23.1.2 of the 4 electronic Application Forms (eAF) is now available. This release v1.23.1.2 is an unplanned hotfix release to fix 3 issues that affected the functioning of the forms. More details on the fixes are available on the release notes. This hotfix release does not affect the Data Exchange Standard (DES).
This new version (v1.23.1.2) of the forms will fully replace the versions 1.23.1.0 and 1.23.1.1 after transitional period, on 4 April 2019. The version of the form should not be changed during an ongoing procedure.
04-02-2019
Version 1.23.1.1 of the 4 electronic Application Forms (eAF) is now available. This release v1.23.1.1 is a bug fix release providing usability improvements and technical defect fixes. This bug fix release does not affect the Data Exchange Standard (DES).
This new version (v1.23.1.1) of the forms will fully replace the version 1.23.1.0 after transitional period, on 4 April 2019. The version of the form should not be changed during an ongoing procedure.
01-02-2019
Update of the eAF user guidance Q&A
The eAF user guidance Q&A has been updated and can be found in the“Guidance documents / Q&A” section.
12-12-2018
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services on Saturday, 15 December and Sunday, 16 December 2018. The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas).
There is no impact to the Variation application form. All other dropdown menus for controlled terminology will continue to work during this maintenance activity.
03-10-2018
The eAF User Guidance Human & Vet Q&A has been updated and can also be found under the section “Guidance documents / Q&A”.
02-10-2018
New versions of the 4 electronic Application Forms (eAF v. 1.23.1.0) and the related release notes are now available. This new version is a hotfix release of the forms and can be used as of 28th September 2018.
The current versions (v1.23.1.0) of the forms can be used and will fully replace the version 1.22.0.1 after a transitional period, on 15 October 2018 as detailed in the eAF Release Milestone plan. The version of the form should not be changed during an ongoing procedure.
23-08-2018
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services on between 08:00-18:00 hrs (UK time) Saturday, 25 August 2018. The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology will continue to work during this maintenance activity.
06-08-2018
The regulatory user guides for the electronic application form for a marketing authorisation (Human and Veterinary) have been updated, see section Guidance documents / Regulatory.
13-07-2018
New versions of the 4 electronic Application Forms (eAF v. 1.23) and the related release notes are now available.
This release includes further integration with OMS for organisation data in proposed and present part of Variation form and other NTA changes. This new version, 1.23 of the forms can be used as of today (13th July 2018) and will fully replace the version 1.22.0.1 after transitional period, on 15 October 2018. The version of the form should not be changed during an ongoing procedure.
01-06-2018
Version 1.23 of the electronic Application Forms (eAFs) will become available on 13 July 2018. The release v1.23 will provide further integration with OMS from SPOR (Organisational/address data) and NTA changes, User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
- The testing by Industry and NCAs will take place
- Industry: from Mon 11/06/18 to Fri 18/06/18
- NCAs*: from Mon 19/06/18 to Fri 22/06/18 (*Based on the eAFs received from Industry)
If you wish to participate, please register by email with eSubprogofficer@ema.europa.eu. A feedback form for consolidated comments will be provided to you following your registration. Please follow Summary of changes, document which shows clearly before and after areas and changes to eAF that focus on test scenarios for current UAT. UAT version of Summary of changes will be sent via email with UAT package. Final version will be published on website later (13 July 2018).
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services on between 18:00-19:00hrs on Thursday, 17 May 2018. The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology will continue to work during this maintenance activity.
15-05-2018
The updated DES documents and XSD files are now available for all 4 forms under the Technical Documents section below. This is in preparation for the scheduled go live of eAF Version 1.23.
13-04-2018
The eAF guidance for how to request new substances and terms has been updated. Please refer to the eAF Term Request Form section below.
07-03-2018
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services on Saturday, 10 March and Sunday, 11 March 2018. The eAF will be impacted for the ATC code field (MAA human and vet and the renewal form) and for the areas where Organisations and Locations are used (however manual input of data is allowed for these areas). There is no impact to the Variation application form. All other dropdown menus for controlled terminology will continue to work during this maintenance activity.
16-02-2018
New versions of the 4 electronic Application Forms (eAF v. 1.22.0.1) and the related release notes are now available. This new version is a hotfix release v1.22.0.1 of the forms and can be used as of today, 16th February 2018. The version of the form should not be changed during an ongoing procedure.
15-02-2018
As of 15 February 2018 version 1.22 of the four electronic Application Forms (eAFs) are mandatory. Older versions of the forms will no longer be supported.
15-12-2017
New versions of the 4 electronic Application Forms (eAF v. 1.22) and the related release notes are now available.
This release includes integration with OMS for organisation data and RMS for reference data.
OMS will provide standardised organisation and location data. (For information, eAF has been integrated with RMS since June 2017.)
This new version, 1.22 of the forms can be used as of today (15th December 2017) and will fully replace the version 1.21 after transitional period, on 15 February 2018. The version of the form should not be changed during an ongoing procedure.
13-12-2017
Please see below the summary of post User Acceptance Testing for eAF v1.22 outcome.
In total 247 comments were received.
We have fixed 62 defects (remaining defects will be assessed and prioritised in future releases).
All submitted change requests will be analysed and prioritised.
| |
|
Change Requests |
Defects |
Defects Addressed |
| eAF |
MAA(Human) |
19 |
38 |
33 |
| |
MAA(Vet) |
2 |
6 |
5 |
| |
Renewal |
9 |
16 |
11 |
| |
Variation |
27 |
22 |
13 |
| |
|
57 |
82 |
62 |
27-11-2017
Essential maintenance to computer applications
The eAF will be impacted by essential maintenance to SPOR data services on Wednesday evening 29th November and Friday 1 December 2017. This maintenance work affects the ATC code field in the MAA human & vet forms and the renewal form. All other dropdown menus for controlled terminology continue working during this maintenance activity. There is no impact to the Variation application form.
22-11-2017
A webinar on the use of OMS and RMS data in eAF will be held on 28 November 2017 at 14:00-16:00 UK time. If you would like to participate in this webinar, please register by email to SPOR-Change-Liaisons@ema.europa.eu. Please note that participation in this webinar is on first come first serve basis as the maximum capacity of the virtual room is 200 participants. The webinar will be recorded and published on the eAF website.
19-10-2017
Version 1.22 of the electronic Application Forms (eAFs) will become available on 15 December 2017. The release v1.22 will provide integration with OMS from SPOR (Organisational/address data), usability improvements and technical defect fixes. User Acceptance Testing (UAT) is planned to support the release of this next version of the forms:
- The testing by Industry and NCAs will take place
- Industry: from Mon 13/11/17 to Fri 17/11/17
- NCAs*: from Mon 20/11/17 to Fri 24 /11/17 (*Based on the eAFs received from Industry)
If you wish to participate, please register by email with eSubprogofficer@ema.europa.eu. A feedback form for consolidated comments will be provided to you following your registration. A kick-off meeting is planned to take place on 13th November 2017 to explain the new features.
05-10-2017
The EMA is launching an updated version of the EudraVigilance on 22 November 2017. In preparation for this update, there will be a downtime period for the Article 57 database (EXVDMP). During the scheduled downtime from 8th-21st of November 2017 it will not be possible to amend or add product/substance data in Article 57. This will impact eAF use as the substance selection for the Initial MAA is linked to EXVDMP. It will not be possible to update substance data for the creation of eAF dataset during this downtime period. The EMA strongly recommends that MAHs, with a MAA date falling during the scheduled downtime (8 to 21 November 2017), carefully review relevant data and ensure that the required substances are available via the eAFs.
12-07-2017
A defect in section 1.4 of the MAA veterinary eAF, affecting the MRL substances has been detected and subsequently fixed. New version v.1.21.0.1 of the MAA veterinary form and the updated release notes are now available.
Defects in section 1 and 3 of the Renewal eAF, affecting the MA Numbers fields and the overages field respectively, have been detected and subsequently fixed. New version v.1.21.0.1 of the Renewal form and the updated release notes are now available.
30-06-2017
A defect in section 1.1 of the MAA human eAF, affecting the PRAC rapporteur field (Centralised Procedure applications) has been detected and subsequently fixed. New version v.1.21.0.1 of the MAA human form and the updated release notes are now available.
Applicants are reminded that for Centralised Procedure a single application form per product should be used – it is not acceptable to provide separate application forms for different strengths/presentations.
20-06-2017
Version 1.21 of the 4 eAFs is now available. The release v1.21 provides usability improvements and technical defect fixes. Details of the changes are available on the updated Release notes. This release implements changes in the Data Exchange Standard (DES) of the MAA-Human and MAA-Vet forms. The updated DES and XSD documents are available under Technical Documents.
15-06-2017
Version 1.21 of the eAFs will be available on 20 June 2017. The release v1.21 provides usability improvements and technical defect fixes. Following a successful User Acceptance Testing (UAT) the release schedule is now available to provide more information on the mandatory use of version 1.21.
08-03-2017
Users of the variation form who are wishing to import data from v1.20.0.3 or earlier are reminded to remove all the scopes in section 3 before exporting the xml in to v1.20.0.5. as the format of the numbering has changed and will cause issue if not deleted before importing to the new version of the form. There is no need to untick the scopes if importing from v1.20.0.4 to v1.20.0.5.

01-10-2016
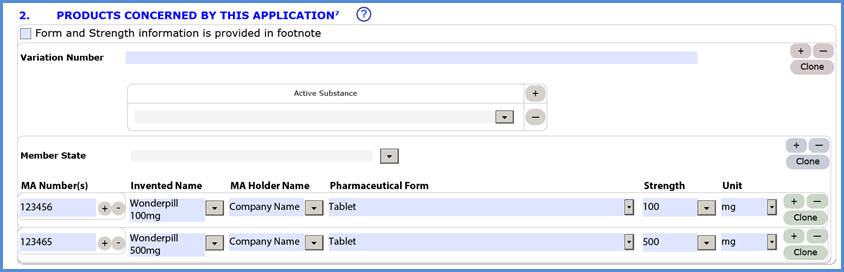
Applicants are reminded that the section 2 of the electronic Application Form (eAF) should be filled in with product details. The use of the footnote is only allowed for those applications that contain complex form and strength data where it may be impossible to enter details in the provided fields, for example complex vaccines. Footnote should not be used for applications where the product details can be entered in section 2. Convenient ‘clone’ function is now available reducing need to type similar information multiple times.
|

