|
23-07-2024
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.15 released to production on 22 July 2024 are now available on PLM Portal Forum and on the PLM Portal eAF web page.
22-07-2024
Updated PLM Portal eAF guide to navigation now available
An updated version of the draft PLM Portal eAF guide to navigation is now available.
17-07-2024
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.14 released to production on 15 July 2024 are now available on PLM Portal Forum and on the PLM Portal eAF web page.
11-07-2024
Invitation to register for the Info Session on PLM Portal eAF - Add package
EMA will host an Information session on 18 July 2024, 11:00 - 12:00 (CEST) to explain and showcase the latest developments and bug fixes deployed in the PLM Portal web-based electronic Application Form (eAF), namely the add package feature. Participants will have the opportunity to ask questions in the last part of the session.
The eAF add package functionality allows the applicants to use the web based eAF for variations adding a new pack size.
The participation is recommended to Industry stakeholders who are filling in application forms for their respective organisations.
Register here, and check the event web page for agenda, presentation and recording.
01-07-2024
Updated PLM Portal eAF FHIR XML Release notes now available
An updated version of the PLM Portal eAF FHIR XML Release notes reflecting the implementation of FHIR XML 2.0.1 in the PLM Portal eAF (released to production on 28 June 2024) are now available on the PLM Portal and in the section of the PLM Portal eAF, Documentation section, together with several updated documents to provide consumers of the FHIR XML message the easiest possible way to upgrade to the eAF XML 2.0.1 or to start the implementation now with the latest version.
18-06-2024
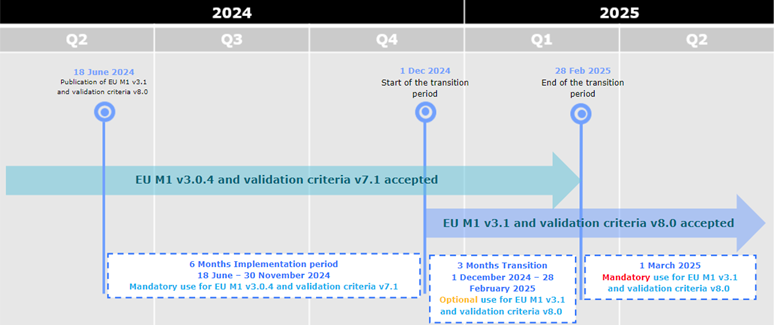
eCTD 3.2.2 new package available
A new version of the EU eCTD M1 Specification, version 3.1, is now published on the eSubmission website
The version 3.1 sees the introduction of a number of changes across the specification, however, more specifically, the introduction of a new annex detailing the list of accepted file formats
(Reminder: the generally accepted file format is the PDF, however, in specific cases, other formats will be exceptionally accepted in the eCTD modules). The changes are reflected in the Release Notes.
The same changes are reflected in the additional files of the EU M1 Implementation Guide package (i.e. eu-envelope.mod, eu-regional.dtd, eu-regional.xsl)
A new version of the validation criteria has been published on the eSubmission website. The version is related to the EU Module 1 Specification version 3.1 and should be used in case of submitting a new sequence according to EU M1 specification v3.1. The new validation criteria will be used for the technical validation for all v3.1 electronic submissions received as of 1 March 2025 to the NCAs and EMA. The changes are reflected in the Release Notes.
During the initial period of 6 months from 18 June 2024 to 30 November 2024, applicants can only submit eCTD format submissions compliant with EU M1 v3.0.4 and validation criteria version 7.1.
From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and validation criteria v7.1 or v8.0 are accepted.
From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.0 are accepted.
13-06-2024
ICH Call for Vendor participation now open
A call for vendor participation is now published on the ICH Official website.
If you are an eCTD v4.0 Tool Vendor and would like to join the eCTD Tool Vendor Group to discuss eCTD v4.0 implementation, please send the following information listed below to ICHM8Vendors@ich.org:
- First Name;
- Last Name;
- Email;
- Company Name;
Please note that your information will be shared as needed with Representatives of ICH Members and Observers involved with ICH eCTD activities.
The Vendor Group kick-off meeting will be held on 17 July 2024, at 7.00 A.M. ET.
The vendor group is solely intended to facilitate collaborative discussion on the eCTD v4.0 Implementation Specification and timelines.
More information can be found on the ICH Official web site: ICH.
AND
ICH eCTD v4.0 package updated
The ICH eCTD v4.0 package has been updated in response to the change requests and/or discussion within the EWG after its initial release. There has been number of updates to the ICH IG and both the Support Documentation and the Orientation Material presentations have been updated. Additionally, as of May 2024, the Implementation Guide and Controlled Vocabulary documents were split into two different packages to enable Controlled Vocabulary Versioning.
More information can be found on the ICH Official web site: ICH.
11-06-2024
Planned maintenance of the PSUR Repository (NCAs only) on 18 June 2024, 18:00 CET
Due to planned maintenance, the PSUR Repository NCA UI and PSUR Repository NCA API will not be available for approximately 1 hour on Tuesday, 18 June 2024, after 18:00 CET. The maintenance is related to a technical upgrade and will not result in any changes of the features and functionalities of the system.
AND
Planned maintenance of the eSubmission systems on 19 June 2024, 18:00 CET
Due to planned maintenance, the following eSubmission systems will not be available on Wednesday, 19 June 2024, between 18:00 and 21:00: Common Repository Web-UI (relevant for NCAs only) and Common Repository API (relevant for NCAs only). During the same interval, the Gateway Filehandler will experience short delays in sending the acknowledgements of the processed submissions.
03-06-2024
PMS Product UI Now Live on the PLM Portal
The PMS Product User Interface (PUI) was launched on 31 May 2024, in read-only mode on Product Lifecycle Management (PLM) Portal. Registered users are now able to view Centrally Authorised Product - (CAP) data in the PUI (Nationally Authorised Product (NAP) data will be available in early Q3 2024).
Please note that the eAF users have now automatically access to the PUI. Consult these guidance documents and join the 3 June 2024 training session to prepare for registration & navigation of PUI.
17-05-2024
Updated PLM Portal Guide to Navigation is now available
Please find an updated version of the draft PLM Portal eAF Guide to Navigation here.
AND
New Paediatric submissions are mandatory via IRIS platform from 4 June 2024
Please note that from 4 June 2024, the following types of new paediatric submissions must be carried out via IRIS:
- Initial paediatric investigation plan (PIP)
- Modification of an agreed PIP
- Product-specific waiver
- Compliance check
- Annual report on paediatric deferred measures
- Confirmation of applicability of a class waiver, or inclusion of an indication within a condition
- Discontinuation of paediatric development.
To ensure a smooth transition to using the IRIS platform, it is essential that applicants prepare well in advance, including registering for IRIS, as described in the following document:
https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/iris-guide-registration-and-rpis_en.pdf
The gateway is open for ongoing paediatric procedures only.
15-05-2024
A new PLM Portal Home page
New, product specific tiles for electronic Product Information (ePI) and the Product Management Service (PMS) Product User Interface have been included in addition to the web-based electronic Application Forms on the PLM Portal's landing page. To ensure users can find and easily access these tools, EMA has updated the landing page of the Portal, which now presents a new look & feel.
The new interface aims to provide more intuitive access to eAF, PMS and ePI release notes, news, knowledge articles.
We would like to invite you to share your feedback on the new landing page in the PLM Portal Forum.
AND
Recommended use of web-based human variations eAF for all CAPs
As anticipated in April 2024 communication and announced in May 2024 at the webinar “Information and Q&A session on updated CAPs in web-based eAF” (recording & presentation available), we have completed the load of updated* Centrally Authorised Products (CAPs) data to PLM Portal web-based eAF on 19 April 2024. Users can now access this new CAP product data directly within the web-based forms.
We are pleased to announce that we recommend the use of the PLM Portal web-based eAF for all CAPs variations starting from 14 May 2024. However, please be aware that there are still some known issues and limitations that we are actively addressing however, these issues do not prevent the use of the web-based eAF. We invite you to consult the 7 May webinar presentation for the complete list of known issues.
If you encounter any issues, please report them via EMA Service Desk, ensuring that you select the correct category.
*Including split & match-merge processes. Please refer to EU IG (Implementation Guide) Chapter 7 for a detailed description of these steps.
26-04-2024
Paediatric submissions to launch on IRIS platform from 4 June 2024
Please note that from 4 June 2024, the paediatric submissions must be carried out via IRIS. For more information please see the announcement.
24-04-2024
Register for the Information and Q&A session on updated CAPs in PLM Portal eAF, 7 May 2024
EMA will host an Information and Q&A session on 7 May 2024, 10:00 - 11:00 (CEST) to explain and showcase the changes in the PLM Portal web-based Human Variations eAF after the updated Centrally Authorised Products (CAPs) load. Participants will have the opportunity to ask questions in the last part of the session.
The participation is recommended to Industry stakeholders working on regulatory affairs of their respective organisations.
Register here, and check the event web page for agenda, presentation and recording, all to be published in due time.
23-04-2024
Successful CAPs data load under review, recommended use of interactive PDF extended
Updated Centrally Authorised Products (CAPs) data has been loaded to web-based (PLM Portal) eAF, where users will be able to see this data in their forms.
Please note that, while CAPs data is available as of 16 April, we strongly recommend that no applications using the PLM Portal web-based eAF is used for submissions at the moment. This is due to the fact that the updated CAPs load has impacted how products appear in application forms (e.g., changes to product names, introduction of new medicinal products through splitting, potential alterations to packages, etc.) and some minor adjustments to the functionalities of the web based eAF are needed.
We suggest using the interactive PDF eAF instead of the web-based eAF for submissions to prevent validation issues and potential delays. The EMA will announce the date from which the use of the PLM web-based eAF form can be resumed.
19-04-2024
Updated VNeeS checker for v3.1.1 now available
An updated version of the VNeeS checker provided by Anses - ANMV is now available.
11-04-2024
Updated eAF v1.26.0.1 (Vet MAA) now available
An updated v1.26.0.1 Vet MAA eAF is now available on the eSubmission website.
A change has been implemented to fix a bug related to the navigation in the form after its finalisation.
It is recommended to use this latest form for new submissions. Please note that there is no version number change and that the release notes will be updated and published in the relevant section of the eAF page.
AND
electronic Application Forms (eAFs) not available for use between 11th and 16th April 2024 due to planned maintenance
Due to planned maintenance activities, the interactive PDF eAFs (human and veterinary variation and MAA forms and the renewal form) and the PLM Portal web-based eAF will be unavailable for use between 11th and 16th April 2024.
Please refer to the news published on 15 March 2024 for the complete details of the planned maintenance and we remind you that we strongly recommend to use the interactive pdf eAF instead of the web-based eAF for submissions at least until 26 April 2024 to prevent validation issues and potential delays.
20-03-2024
Planned maintenance of the eSubmission systems on 25 March 2024, 18:00 CET
Due to planned maintenance, the following eSubmission systems will not be available on Monday 25 March 2024, between 18:00 and 21:00: Gateway XML delivery file user interface and Common Repository Web-UI (relevant for NCAs only).
The maintenance includes the introduction of a new field in the eSubmission Gateway XML delivery file user interface (EPITT number for pam-sda submissions), and other small bug fixes and improvements.
Previously created delivery files will not continue to work, please use the new version of the Gateway XML delivery file after the maintenance has completed.
Updated documentation will be published on the relevant pages of the eSubmission website.
For any further information, please contact EMA Service Desk.
15-03-2024
electronic Application Forms (eAFs) not available for use between 11th and 16th April 2024 due to planned maintenance
Due to planned maintenance activities, the interactive PDF eAFs (human and veterinary variation and MAA forms and the renewal form) and the PLM Portal web-based eAF will be unavailable for use between 11th and 16th April 2024.
Additionally, as announced in December 2023, the PLM Portal web-based eAF will be affected by the data load of Centrally Authorised Products (CAPs) and Nationally Authorised Products (NAPs) into Product Management Service (PMS) as well as the simultaneous load of updated* CAPs to web-based eAF.
This load of data into PMS is a necessary step in preparation to the forthcoming launch of the Product User Interface view and later edit functions as well as making NAPs data available for the PLM Portal web-based eAF.
The data load will take place from 11 to 16 April 2024. During this timeframe, the interactive PDF eAF and the PLM Portal web-based eAF will experience a downtime.
Please note that, during the preparation for the updated* CAPs load in the PLM Portal web-based eAF, the match-merge** operation will result in:
- changes to product names;
- introduction of new medicinal products through splitting**;
- potential alterations to packages.
These changes will impact how products appear in application forms. Therefore, we strongly recommend, with immediate effect, that no applications that might require any update of the eAF, during or after the product upload, are submitted to EMA using the PLM Portal web-based eAF. We strongly recommend to use the interactive pdf eAF instead of the web-based eAF for submissions at least until 26 April 2024 to prevent validation issues and potential delays.
Please note that if you have already submitted a web-based eAF, or it is expected that there will be no need to update the form and/or the procedure will conclude prior to the downtime, you can continue using the web-based eAF.
Please note that, except for the downtime period, the web-based eAF is expected to remain accessible to applicants to familiarise themselves with changes to the products, and for training purposes.
*Including split & match-merge processes. Please refer to EU IG (Implementation Guide) Chapter 7 for a detailed description of these steps.
**The “Match-merge” process serves to include data from XEVMPD to products already released in PLM Portal. The “split” process serves to make released products ISO-IDMP compliant. Both processes are explained in detail in EU IG Chapter 7
15-03-2024
Register for the eCTD v4.0 Vendor Workshop on 27 March 2024
The electronic Common Technical Document (eCTD) is the standard norm for industry submissions for over 20 years and currently implemented by regulators and industry in version 3.2.2. Version 4.0 of eCTD was published in December 2015 with its most recent Implementation Package v1.5 endorsed at the May 2022 ICH meeting.
This workshop, organised and facilitated by the eCTD v4.0 SME Group (EMA, NCAs and Industry), is intended for all eCTD Vendors that are interested in better understanding changes to the EU regional eCTD Specification. The event aims at fostering a discussion around the key sections of the new version of the Implementation Guide, the technical understanding of the implementation of the new standard, including the grouped submissions, document re-use and controlled vocabularies. At the moment, the focus will be on the Centralised Procedures only.
Moreover, during the workshop, the scope and timeline of the pilot, as well as the timeline for the updates of the Implementation Guide, will be discussed.
This session also offers an invaluable opportunity for participants to ask questions and provide feedback on pertinent matters.
Register for this workshop.
After registration you will receive a confirmation e-mail with the link and password to access the workshop. Please make sure to save those to be able to participate to the workshop.
15-03-2024
Planned maintenance of the Common Repository Torrent in the evening of 21 March 2024
Due to planned maintenance, the use of the Torrent files via client to download the content will not be possible on Thursday 21 March 2024, between 18:00 and 21:00. For any further information, please contact EMA Service Desk.
14-03-2024
eCTD v4.0 EU M1 Implementation Guide - draft Version 1.1 now available
A new draft version of the eCTD v4.0 EU M1 Implementation Guide is now available here for consultation. Future versions (together with EU Controlled Vocabularies and other annexes) will be published and announced on the eSubmission website.
29-02-2024
Updated eAF v1.26.0.1 (Vet MAA) now available
An updated v1.26.0.1 Vet MAA eAF is available starting with 29 February 2024, 18:00 CET.
A change has been implemented to add "United Kingdom (Northern Ireland)"" in the Member state selection in sections 2.4.1 and 2.4.4.
It is recommended to use this latest form for new submissions. Please note that there is no version number change and that the release notes will be updated and published in the relevant section of the eAF page.
20-02-2024
PLM Portal eAF and PMS - FAQs and Q&A documents updated
The PLM Portal eAF and PMS teams are pleased to announce that the updated Frequently Asked Questions (FAQs) on eAF and PMS and Questions and Answers (Q&A) Joint eAF and PMS are now available here.
02-02-2024
Updated PLM Portal eAF FHIR XML Release notes now available
An updated version of the PLM Portal eAF FHIR XML Release notes reflecting the implementation of FHIR XML 2.0.0 in the PLM Portal eAF (to be released to production on 30 April 2024) are now available on the PLM Portal and in the section of the PLM Portal eAF, Documentation section, together with several documents to provide consumers of the FHIR XML message the easiest possible way to upgrade to the eAF XML 2.0.0 or to start the implementation now with the latest version.
The new version includes the latest official FHIR version “Release #5 (5.0.0): http://hl7.org/fhir/”. This replaces the current version FHIR Release #5: Draft Ballot (4.6.0): http://hl7.org/fhir/2021May/ which will remain online for approximately 3 months to give consuming systems time to adapt to the change.
02-02-2024
User Experience of the current PDF eAFs completion on the PLM Portal
The electronic Application Form (eAF) Product Team would greatly appreciate to receive your feedback on your current experience in terms of time spent in filling in the interactive pdf electronic Application Form (eAF) for Variations for Centrally Authorised Products (CAPs) and Nationally Authorised Products (NAPs), and Initial Marketing Authorisation.
It is acknowledged that different types of procedures (e.g. Type IA, Type II applications, worksharing, and groupings) may require varying time commitments. We would therefore appreciate if, in your answer, you could provide the average time spent and resources used to fill in the forms.
Your feedback will provide us an indication on the current performance requirements and will be our starting point for improving future user experience.
You can find the questionnaire to fill in at the following link:
https://ec.europa.eu/eusurvey/runner/Eusurvey_PLMPortal_UX_eAFcompletion
Please note that this survey should not take more than 5 minutes to complete, and the responses will remain anonymous.
We kindly ask you to respond to the survey by Thursday 29 February 2024.
Please send any question to plm.valuestream@ema.europa.eu or post them via the PLM Portal Forum.
18-01-2024
A new version of the eSubmission Gateway XML delivery file for veterinary Variations Requiring Assessment (VRA) now available
An updated version of the eSubmission Gateway XML delivery file user interface for veterinary VRA submissions is available starting with 17 January 2024, 18:00 CET.
This update introduced a split of the submission type VRA to 4 different submission types according to the Timetable as per defined in the Variation Classification document.
The users should note that they should always select the standard timetable (E/S/R) for each scope, regardless of a different TT having been agreed.
For grouped VRA, in the delivery file, the applicant should select the longest timetable applicable to any of the included variations in the application.
Updated release notes and user guidance are published in the relevant section.
Users should note that the delivery files created prior to the new release will not work after the go live.
15-01-2024
A new version of the eSubmission Gateway XML delivery file for veterinary Variations Requiring Assessment (VRA) is planned for release in the evening of 17 January 2024
An updated version of the eSubmission Gateway XML delivery file user interface for veterinary VRA submissions is planned for release in the evening of 17th January 2024. This update will introduce a split of the submission type VRA to 4 different submission types according to the Timetable as per defined in the Variation Classification document.
The users should note that they should always select the standard timetable (E/S/R) for each scope, regardless of a different TT having been agreed.
For grouped VRA, in the delivery file, the applicant should select the longest timetable applicable to any of the included variations in the application.
Users should note that the delivery files created prior to the new release will not work after the go live.
And
A new version of the eSubmission Gateway XML delivery file for SEND packages now available
An updated version of the eSubmission Gateway XML delivery file user interface is available starting with 11 January 2024, 18:00 CET. This update introduced in the delivery file for Human submissions the option to specify if there is a “SEND Data package Included”.
Updated release notes and user guidance are published in the relevant section.
Users should note that the delivery files created prior to the new release will not work after the go live.
10-01-2024
Opportunity to submit SEND data packages with new Market Authorisation Applications
From January 2024, EMA is launching a proof-of-concept study to evaluate the added value of using SEND data in the evaluation of new Marketing Authorisation Applications. Applicants are encouraged to submit their SEND data packages, in addition to the eCDT format, as part of their MAA submission. The SEND package must be provided outside the eCTD, inside the working documents folder to avoid eCTD technical validation failure.
An updated version of the eSubmission Gateway XML delivery file user interface will be available starting with 11 January 2024, 18:00 CET. This update introduces in the delivery file for Human submissions the option to specify if there is a “SEND Data package Included”.
Users should note that the delivery files created prior to the new release will not work after the go live.
What is SEND?
SEND is the Standard for Exchange of Nonclinical Data between organisations, which provides a standardised format for the submission of nonclinical data to regulatory bodies.
SEND was created by the Clinical Data Interchange Standards Consortium (CDISC) in 2002 to execute on the Study Data Tabulation Model (SDTM) for the submission of nonclinical studies. A SEND dataset package contains the SEND datasets (.xpt files), the Nonclinical Study Data Reviewer's Guide (nsdrg.pdf), and the Define XML Document (define.xml).
Why a proof-of concept?
Standardisation of data presentation has been shown to significantly reduce the time regulators require for reviewing the non-clinical data packages. In this proof-of-concept study, we will evaluate whether using SEND data in the assessment of the non-clinical dossier will lead to improved and more consistent quality of assessments, to more science-driven questions to Applicants, and to faster completion of the non-clinical dossier assessment.
Any questions? Email us at send@ema.europa.eu
21-12-2023
eCTD v4.0 Vendor Workshop Survey
EMA, together with the eCTD v4.0 EU Subject Matter Experts group, is planning a Vendor Workshop in Q1 2024. To assess the availability, interest and further topics for discussion, the Vendors are invited to complete the eCTD v4.0 Vendor Workshop survey by end of day 15 February 2024.
20-12-2023
Update on web-based Human variations electronic Application Forms (eAFs) implementation
As anticipated in October 2023, the electronic Application Form (eAF) and Product Management Service (PMS) teams would like to provide you with an update on the progress of the web-based Human variations eAF implementation on the Product Lifecycle Management (PLM) Portal
During Q4 2023, we completed the load and evaluation of PMS data in PMS User Acceptance Testing (UAT) environment, after which we proceeded to testing of PMS data in the PLM Portal. While performing these activities, we observed the following:
- Bugs in PMS related to match-merge* of Centrally Authorised Products (CAPs);
- Technical issues with the eAF preventing testing;
- System performance improvements required for the PLM Portal.
Taking into consideration these findings, we have made the necessary adjustments to planning. Kindly find here a visual representation of the updated plan.
Please note that Alpha UAT for Product User Interface (UI) data quality is still ongoing and proved successful thus far, as no blocking bugs have been identified.
Key points of the updated plan:
Q1 2024:
- Deployment in production of new eAF features (e.g., clone application, add package, rename application) developed in Q4 2023.
- Consolidation of all PLM portal products' development (eAF, Product UI, ePI) under a single service provider.
Q2 2024:
- Release of all CAPs & NAPs in the PMS database. Simultaneously, release of updated** CAPs in eAF;
- Delivery of PMS Application Programming Interface (API) - with CAPs and Nationally Authorised Products (NAPs) available in view-only mode - and Product UI - with CAPs available in view-only mode and NAPs not yet viewable.
Please note that, during the preparation for the updated** CAPs load in eAF in Q2 2024, the match-merge* operation will result in:
- changes to product names;
- introduction of new medicinal products through splitting*;
- potential alterations to packages;
This will impact how products appear in application forms. Therefore, we will communicate in due course the 3-week period where it is advisable to use the interactive eAF (PDF) instead of the web-based eAF for submissions to prevent validation issues and potential delays. Please note the web-based eAF will remain accessible to applicants to familiarise themselves with it and for training purposes.
Q3 2024:
- Performance improvements and internal testing
Q4 2024:
- Release of NAPs in the eAF
The updated planning does not foresee in 2024:
- external testing of the eAF UAT
- consequently, no transition start towards mandatory use of the Human variations web-based eAF. However, we would recommend the use of the web-based eAF for CAPs submissions following the updated** CAPs release.
Please send any questions to eSubProgofficer@ema.europa.eu or post them via the PLM Portal Forum.
*The “Match-merge” process serves to include data from XEVMPD to products already released in PLM Portal. The “split” process serves to make released products ISO-IDMP compliant. Both processes are explained in detail in EU IG Chapter 7
**Including split & match-merge processes. Please refer to EU IG Chapter 7 for a detailed description of these steps.
07-12-2023
Updated eAF v1.26.0.0 (Human MAA) and v1.26.0.1 (Vet MAA) now available
Updated v1.26.0.0 Human MAA eAF is now available on the eAF website. The form is ready for immediate use. The change implemented in this version is a minor bug fix (related to Annex 5.19).
It is strongly recommended to use this latest version (document properties date 27.10.2023). Please note that there is no version number change and that the release notes are published in the relevant section of the eAF webpage.
Updated v1.26.0.1 Vet MAA eAF is now available for immediate use.
The changes in this version relate to label and text changes only. Additionally, the version number has updated from v1.26.0.0 to v1.26.0.1.
The version 1.26.0.1 can be used immediately, and it is strongly recommended that it will be used for all new applications as soon as possible, however, the one-month transitional period will run until 8th of January 2024 after which the use of version 1.26.0.1 for the Veterinary MAA form will become mandatory.
The version 1.26.0.0 has now been removed from the eAF website however users can continue to submit applications using this version until 7th of January 2024.
Please note that as there are no technical changes, it is possible to import the xml from version 1.26.0.0 into version 1.26.0.1 to avoid any need for manual effort.
Applicants are reminded that the version of the form should not be changed during an ongoing procedure, please note that if you need to provide an updated form for a procedure that has started prior to 8th of January, you should use the previous version.
Updated release notes are published in the relevant sections of the eAF webpage.
05-12-2023
Survey for eSubmission website (open until Monday, 18 December 2023)
In light of an ongoing application study at the European Medicines Agency, we are gathering feedback on the eSubmission website.
By completing this short 2-3 minutes survey, you help us to understand if the website addresses your user needs.
May we kindly ask you to complete this survey by Monday, December 18th? The link to the survey can be found here: eSubmission User Survey.
We highly appreciate your input.
And
05-12-2023
Survey for PSUR Repository (open until Monday, 18 December 2023)
In light of an ongoing application study at the European Medicines Agency, we are gathering feedback on the PSUR Repository.
By completing this short 2-3 minutes survey, you help us understand if the application addresses your user needs.
May we kindly ask you to complete this survey by Monday, December 18th? The link to the survey can be found here: PSUR Repository User Survey.
We highly appreciate your input.
01-12-2023
New submission unit to be used for requesting a Re-examination of an CHMP Opinion
An updated version of the eSubmission Gateway XML delivery file user interface will be available starting with 1 December 2023, 18:00 CET. This update introduces in the delivery file for Human submissions the option to choose "Re-examination" submission unit for various submission types. Use this unit for requesting re-examination of opinion for MAA, extension, Type II variation, renewal and annual re-assessment as well as Referral procedures
Please note that regulatory guidance referring to how to send re-examination requests will be updated in the following period. Re-examination requests should be submitted via the eSubmission (Syncplicity) Gateway using eCTD format where required for the procedure type.
30-11-2023
Updated version of the eAF v1.26.0.0 (variation and renewal)
An updated version 1.26.0.0 of the Variation and Renewal (both Human and Veterinary) eAF is available starting with 28 November 2023, 18:00 CET.
A change has been implemented to allow Non-Current terms to be selected in the "Pharmaceutiacal form" lists.
It is recommended to use this latest form for new submissions. Please note that there is no version number change and that the release notes were updated and published in the relevant section of the eAF page.
28-11-2023
eCTD EU Module 1 Specification proposed changes now open for public consultation (Deadline: 12 January 2024)
The Human Harmonisation Group has analysed the change requests received upon opening the eCTD EU Module 1 Specification for review and is now publishing the draft documents for public consultation:
The eCTD EU Module 1 Specification contains a summary of the proposed changes in the Document control table, and all the amendments are highlighted throughout the text with the track changes feature.
The eCTD EU Validation Criteria contains a summary of the proposed changes in the Document control tab.
Please submit your questions and comments via email to EUM1Spec@ema.europa.eu by the end of the day 12 January 2024.
After consolidating the public comments, the final versions of the documents and all the relevant files (including the Release notes, Package, Checksums) will be published on the eSubmission website. The date of entering into force will be announced at the time of the publication and will allow 9 months for the implementation of the changes.
28-11-2023
Updated PLM Portal eAF Release notes now available
An updated version of the PLM Portal eAF Release notes reflecting bug fixes and updates to web eAF made in the version 1.0.1.12 released to production on 27 November 2023 are now available on PLM Portal Forum and on the PLM Portal eAF web page.
20-11-2023
Invitation to register for the Product Lifecycle Management Value Stream Deep-Dive Webinar, 30/11/23 h. 14:00-16:00 (CET)
EMA is pleased to announce a public webinar (event page on EMA website) for medicines developers and regulators eager to gain deeper insights into what the Agency's Product Lifecycle Management (PLM) value stream is working on and its goals. The event will take place on 30 November, 14:00 - 16:00 (CET).
This webinar aims to show the interconnections between the various digital products being delivered. In addition, it will outline the approach taken to enhance product lifecycle management technology to enable more efficient and effective medicine regulation.
About PLM:
PLM is one of three value streams covering the product lifecycle implemented by the Agency as part of its Agile transformation. PLM aims to digitally transform and optimise regulatory procedure management as well as data submission and reuse throughout the product lifecycle. The value stream works with EMA partners and stakeholders to deliver systems and services to manage the authorisation and lifecycle of medicinal products and medical devices within the Agency's remit for the ultimate benefit of public and animal health in the EU.
About the Webinar:
During the webinar, the team will share the value stream's vision and roadmap towards a data centric target operating model for end-to-end data capture, processing and re-use, as well as how PLM's products and solutions contribute to this vision.
Featured PLM Products and Solutions:
- Solutions for Human & Veterinary medicinal products:
- Solutions for Human medicinal products:
- Solutions for Veterinary medicinal products:
What's in it for you:
This webinar presents a valuable opportunity to enhance your understanding of the work of the value stream and stay informed about the latest developments in this area, ask questions and interact with the responsible EMA teams.
Registration & Contact details:
Interested stakeholders are invited to kindly register at the following link.
Please note registration will remain open until the start of the webinar.
You have the opportunity to send your questions in advance via Slido.com using the specific event code: #PLMVS-QA.
Should you have any questions or require further information, please feel free to contact us at plm.valuestream@ema.europa.eu.
14-11-2023
Updated PLM Portal eAF FHIR XML Release notes now available
An updated version of the PLM Portal eAF FHIR XML Release notes reflecting the implementation of FHIR XML 1.2.0 in the PLM Portal eAF (to be released to production on 21 November 2023) are now available on PLM Portal Forum
23-10-2023
Updated VNeeS checker for v3.1 now available
An updated version of the VNeeS checker provided by Anses - ANMV is now available here
19-10-2023
Updated variation Conceptual Data Model
The conceptual data model for the variation form has been updated and is now available here
06-10-2023
Updated version of the VNeeS specification v3.1 now available
The guideline on eSubmissions for Veterinary products version 3.1 has now been published. It will enter into force on 1 November 2023 (see links under section “Current Guidance”).
The update is related to a best practice criterion in the technical validation checklist for the additional information folder (“add-info”). In case you will be using a checker tool not yet aligned with this new best practice requirement, for relevant submissions like SRPs, in case of doubt, we recommend to verify the path length in the add-info folder with the publicly available VNeeS checker tool to avoid potential technical blocks during dossier upload and thus circumvent the need for last minute changes of your submission.
06-10-2023
eSubmission Gateway XML delivery file update for Article 18 submissions
An updated version of the eSubmission Gateway XML delivery file user interface is now available. This update has introduced a small change to the delivery file for Human submissions (option to choose Article 18 as submission type).
The details on the changes will soon be provided in the updated release notes and the updated user guide. More information on Article 18 can be found
here.
05-10-2023
Update on web-based electronic Application Forms (eAFs) implementation
The electronic Application Form (eAF) and Product Management Service (PMS) teams would like to provide you with a comprehensive update on the progress of the web-based Human variations eAF implementation on Product Lifecycle Management (PLM) Portal.
The following milestones are now for Q1 2024:
- Release of split* (i.e. IDMP compliant) Centrally Authorised Products (CAPs) for use in the variations eAF;
Release National Authorised Products (NAPs) for use into the Human variations eAF;
Introduction of the Product Data Management User Interface (UI), offering users seamless access to view their product data available in the Product Management Services (PMS) database.
As we advance, we remain committed to transparency and will provide a comprehensive update on these targets at the end of Q4 2023, evaluating the quality of both the system and available data.
Currently, our primary focus is ensuring quality of data and system's performance to prepare a transition-ready version of the web-based Human variations eAF. To begin the transition phase towards mandatory use of web-based Human variations eAFs, the following key steps are required:
- Implementation of missing features.
- Resolving pending issues that prevent users from completing a form.
- Releasing all products (NAPs and split* (i.e. IDMP compliant) CAPs) from the Product Management System (PMS) to the PLM portal in the production environment.
- Capability to keep PMS data synchronised with existing databases.
- Ensuring system stability.
As previously committed, any announcement concerning the starting date of the formal transition period will follow User Acceptance Testing (UAT) of the system and the subsequent addressing of any critical issues. This is explained in this slide.
The primary objective remains to implement web replacements of interactive PDF eAFs, enabling user-friendly capture and handling of variations, marketing authorisations, and renewals application data for both applicants and regulators. This transition ensures consistency across IT systems and guarantees the availability of high-quality ISO IDMP compliant information.
For further details on the implementation progress and challenges, and the opportunity to ask questions, interested parties are invited to the joint eAF-PMS webinar for 6 November 2023 (13:30 - 15:00 (CEST)). During this session, the eAF team will also demonstrate the anticipated "add package" and "clone application" features that will be released on the PLM Portal for eAF. Please find here the registration link.
We also recommend that anyone with an interest in the development of eAF and PMS watch the relevant sections of the Q3 System Demo recording, available on EMA's website.
Please send any questions to eSubProgofficer@ema.europa.eu or via the PLM Portal Forum.
*CAPs migrated from SIAMED not following ISO IDMP structure. For this reason, they have undergone a further step in the data migration to PMS in addition to the match and merge protocol.
04-10-2023
Updated user guide for the eAF for MAA available on the CMDh website
A new version of the joint EMA/CMDh User guide for the electronic application form for a Marketing Authorisation is available on the CMDh website.
For the UK, as from 1.1.2021, EU Law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland/NI.
|

