|
28-11-2025
eCTD 3.2.2 - EU M1 v3.1.1 and Validation Criteria v8.2
mandatory from 1st December
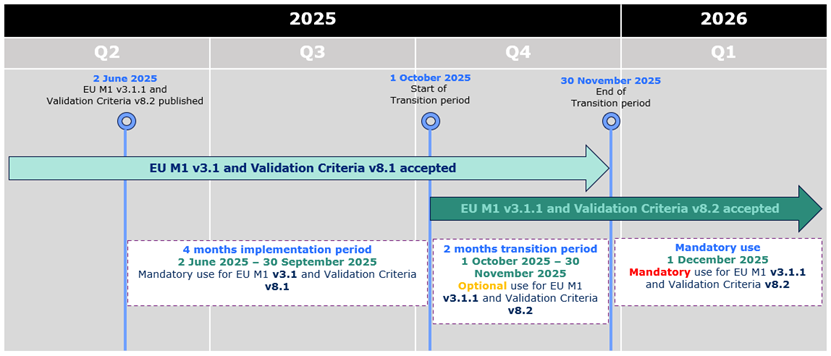
As previously announced, the updated EU M1 specification v3.1.1 and the
related Validation Criteria v8.2 were accepted from 1st
October 2025, and
the mandatory use of the updated specification and validation criteria will
commence on 1st December 2025.
The changes are reflected in the Release Notes.
16-09-2025
eCTD 3.2.2 - EU M1 v3.1.1 and Validation Criteria v8.2 accepted from 1st October
As previously announced, the updated EU M1 specification v3.1.1 and the related
Validation Criteria v8.2 are accepted from 1st October 2025 kicking
off 2 months transitional period during which both the current and the new specification and
validation criteria can be used. The mandatory use of the updated specification
and validation criteria will commence on 1st December 2025.
The changes are reflected in the Release Notes.
02-06-2025
eCTD 3.2.2 - new package available
A new version of the
EU eCTD M1 Specification, version 3.1.1
,
is now published on the
eSubmission website
.
The version 3.1.1 sees the introduction of a small number of changes in the
specification, related to the tracking table for EDQM and new examples of product
numbers and procedure numbers for work-sharing and super-grouping.
The changes are reflected in the
Release Notes
.
A new version of the
validation criteria v8.2
has been published on the
eSubmission website. The version is related to the
EU Module 1 Specification version 3.1.1 and should be used in case of submitting a new
sequence according to EU M1 specification v3.1.1. The new validation criteria will
be used for the technical validation for all v3.1.1 electronic submissions received
as of 1 December 2025 to the NCAs and EMA.
The changes are reflected in the
Release Notes.
A new version of the
Harmonised guidance
eCTD 6.0.1 was published together with the new version of the specification and the validation criteria
Note: The Util files remain the same
During the initial period of 4 months from 2 June 2025 to 30 September 2025,
applicants can only submit eCTD format submissions compliant with EU M1 v3.1 and validation criteria version 8.1.
From 1 October 2025 eCTDs compliant with EU M1 v3.1 or v3.1.1 and validation criteria v8.1 or v8.2 are accepted.
From 1 December 2025 only eCTDs compliant with EU M1 v3.1.1 and validation criteria v8.2 are accepted.
02-04-2025
eCTD 3.2.2 - validation criteria 8.1 to be updated
In the most recent validation criteria (version 8.1) for EU eCTD M1 specification, the rules 15.11 and 15.12 referring to the tracking table being mandatory for all submission types were updated. However, these rules do not apply to EDQM submissions; a new version of the EU eCTD M1 package will be published at a later stage.
In the meantime, for EDQM submissions, you can submit the packages even if the validation does not pass for rules 15.11 and 15.12.
21-01-2025
eCTD 3.2.2 - validation criteria 8.1 to be updated
In the most recent validation criteria (version 8.1) for EU eCTD M1
specification, the
rules 15.11 and 15.12 referring to the tracking table
being mandatory for all submission
types were updated. However, these rules do not apply to EDQM submissions, and therefore
a further communication and potential update of the EU eCTD M1 package will be
published. In the meantime, for EDQM submissions, you can use the previous validation
criteria package (7.1).
28-10-2024
Minor update to the published EU Validation criteria
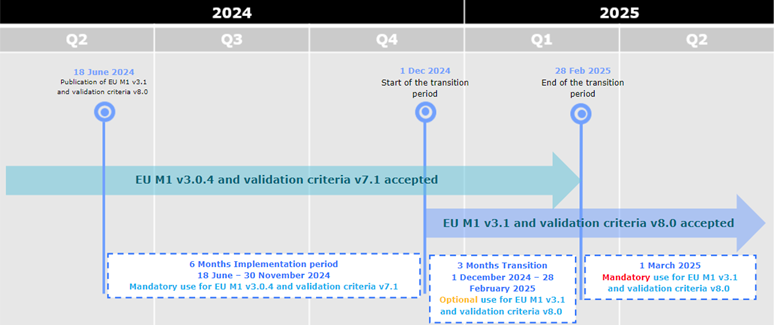
An updated version of the validation criteria to add further clarification the has been published on the eSubmission website. The version is related to the EU Module 1 Specification version 3.1 and should be used in case of submitting a new sequence according to EU M1 specification v3.1. The new validation criteria will be used for the technical validation for all v3.1 electronic submissions received as of 1 March 2025 to the NCAs and EMA. The changes are reflected in the Release Notes.
From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and validation criteria v7.1, 8.0 or v8.1 are accepted.
From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.1 are accepted.
18-06-2024
eCTD 3.2.2 new package available
A new version of the EU eCTD M1 Specification, version 3.1, is now published on the eSubmission website
The version 3.1 sees the introduction of a number of changes across the specification, however, more specifically, the introduction of a new annex detailing the list of accepted file formats
(Reminder: the generally accepted file format is the PDF, however, in specific cases, other formats will be exceptionally accepted in the eCTD modules). The changes are reflected in the Release Notes.
The same changes are reflected in the additional files of the EU M1 Implementation Guide package (i.e. eu-envelope.mod, eu-regional.dtd, eu-regional.xsl)
A new version of the validation criteria has been published on the eSubmission website. The version is related to the EU Module 1 Specification version 3.1 and should be used in case of submitting a new sequence according to EU M1 specification v3.1. The new validation criteria will be used for the technical validation for all v3.1 electronic submissions received as of 1 March 2025 to the NCAs and EMA. The changes are reflected in the Release Notes.
During the initial period of 6 months from 18 June 2024 to 30 November 2024, applicants can only submit eCTD format submissions compliant with EU M1 v3.0.4 and validation criteria version 7.1.
From 1 December 2024 eCTDs compliant with EU M1 v3.0.4 or v3.1 and validation criteria v7.1 or v8.0 are accepted.
From 1 March 2025 only eCTDs compliant with EU M1 v3.1 and validation criteria v8.0 are accepted.
28-11-2023
eCTD EU Module 1 Specification proposed changes now open for public consultation (Deadline: 12 January 2024)
The Human Harmonisation Group has analysed the change requests received upon opening the eCTD EU Module 1 Specification for review and is now publishing the draft documents for public consultation:
The eCTD EU Module 1 Specification contains a summary of the proposed changes in the Document control table, and all the amendments are highlighted throughout the text with the track changes feature.
The eCTD EU Validation Criteria contains a summary of the proposed changes in the Document control tab.
Please submit your questions and comments via email to EUM1Spec@ema.europa.eu by the end of the day 12 January 2024.
After consolidating the public comments, the final versions of the documents and all the relevant files (including the Release notes, Package, Checksums) will be published on the eSubmission website. The date of entering into force will be announced at the time of the publication and will allow 9 months for the implementation of the changes.
26-10-2022
eCTD EU Module 1 Specification now open for review and all stakeholders are invited to submit change requests on the specification - deadline extended until 1st November 2022
The Human Harmonisation Group has decided to open the eCTD EU Module 1 Specification for review and all stakeholders are invited to review the list of submitted change requests and, to submit new change requests on the specification.
Please find a list of submitted change requests and the template to submit any new change requests to the current version of the eCTD EU M1 specification. Please submit your change requests via email to EUM1Spec@ema.europa.eu by the end of the day 1st of November 2022.
26-09-2022
eCTD EU Module 1 Specification now open for review and all stakeholders are invited to submit change requests on the specification
The Human Harmonisation Group has decided to open the eCTD EU Module 1 Specification for review and all stakeholders are invited to review the list of submitted change requests and, to submit new change requests on the specification.
Please find a list of submitted change requests and the template to submit any new change requests to the current version of the eCTD EU M1 specification. Please submit your change requests via email to EUM1Spec@ema.europa.eu by the end of the day 25th of October 2022.
Updated EU M1 eCTD Specification
The EU eCTD Module 1 Specification has been updated to v3.1.1.
Release notes with practical information on changes are provided below.
There are no changes to the DTD (version 3.1 of the DTD remains valid)
and the validation criteria was updated to v8.2 (release notes are also published).
During the initial period of 4 months from 2 June 2025 to 30 September 2025,
applicants can only submit eCTD format submissions compliant with EU M1 v3.1 and validation criteria version 8.1
From 1 October 2025 eCTDs compliant with EU M1 v3.1 or v3.1.1 and validation criteria v8.1 or v8.2 are accepted.
From 1 December 2025 only eCTDs compliant with EU M1 v3.1.1 and validation criteria v8.2 are accepted.
| |
EU M1 v1.4.1 |
EU M1 v2.0 |
EU M1 v3.0.1 |
EU M1 v3.0.3 |
EU M1 v3.0.4 |
EU M1 v3.1 |
EU M1 v3.1.1 |
| Status |
For reference only |
For reference only |
For reference only |
For reference only |
For reference only |
Can be used until 30 November 2025. EU M1 v3.1.1 mandatory starting with 1 December 2025
|
Approved
(Optional use from 1 October 2025)
Mandatory use from 1 December 2025 |
| Release Notes |
View Specification Release Notes
View Annex Release Notes |
View |
View - 12.7.2016 |
View 16.11.2017 |
View Specification Release Notes including practical information |
View Specification Release Notes |
View Specification Release Notes |
| Specification |
View
|
View |
View - 17.05.2016 |
View |
View April 2021 |
View June 2024 |
View June 2025 |
| Annex |
View |
View |
View |
View |
View |
View |
View |
| Accepted file formats |
|
|
|
|
|
Accepted file formats May 2024 |
Accepted file formats May 2024 |
| DTD |
Download |
|
v.3.0.1 |
v.3.0.1 |
v.3.0.1 |
v.3.1 |
v.3.1 |
| Examples |
Download |
|
|
|
|
|
|
| Package |
Download |
Download |
Download - 06.06.2016 |
Download - 06.06.2016 |
Download - 06.06.2016 |
Download - June 2024 |
Download - June 2024 |
| Implementation Guide |
|
Download |
Download - 12.7.2016 |
Download - 06.06.2016 |
For immediate implementation |
Download |
Download |
| Checksum of |
|
|
|
|
|
|
|
| eu-regional.dtd |
91654e96e3bafc5e89df7f892477b246 |
9dc9debb051a9e5762fc19a1f55132da |
290503BF171E7E2E80EF90F0BDE5D91E |
290503BF171E7E2E80EF90F0BDE5D91E |
290503BF171E7E2E80EF90F0BDE5D91E |
f8e473246d58499f9ffff8e51a32380d |
f8e473246d58499f9ffff8e51a32380d |
| eu-envelope.mod |
664a76e3f31a9553d3375d3b21815904 |
0ebe02fcdff7e40eff94a88074ca21 |
D0727AE0FB68B19EDAE49AB9E2E22A4A |
D0727AE0FB68B19EDAE49AB9E2E22A4A |
D0727AE0FB68B19EDAE49AB9E2E22A4A |
cf059a8f48c68fe4ce9ad2947ab854ca |
cf059a8f48c68fe4ce9ad2947ab854ca |
| eu-leaf.mod |
2e976bc60658a964affa5026369a371e |
23b854174e61c68044b9f53c0009af95 |
23B854174E61C68044B9F53C0009AF95 |
23B854174E61C68044B9F53C0009AF95 |
23B854174E61C68044B9F53C0009AF95 |
23b854174e61c68044b9f53c0009af95 |
23b854174e61c68044b9f53c0009af95 |
| eu-regional.xsl |
54f9889822e1d08cc23b902fc6a66aaa |
bf40b657579356c7b4d6c866625d8171 |
0107179C3739EBBD6B00CE492FE6E1E7 |
0107179C3739EBBD6B00CE492FE6E1E7 |
0107179C3739EBBD6B00CE492FE6E1E7 |
474f3fe1fdadb31c74477793e060383d |
474f3fe1fdadb31c74477793e060383d |
For the UK, as from 1.1.2021, EU Law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland/NI.
|

