News
Previous news available Here
15-12-2025
Go-live announcement for EU eCTD v4.0 optional use for CAP new MAA
The European Medicines Agency (EMA) is pleased to announce that from 22 December 2025, applicants may optionally submit new Marketing Authorisation Applications (MAAs) for Centrally Authorised Products (CAPs) in eCTD v4.0.
Given the need to ensure that enhanced support is provided and that the assessment team is informed, applicants must contact the EMA eCTD v4.0 team (eCTD4consultation@ema.europa.eu) prior to any eCTD v4.0 submission.
Note that the Agency is closed from the 23rd of December 2025 until the 2nd of January 2026 included.
The eCTD v3.2.2 remains accepted during the optional-use period. Additional updates will be provided in the course of 2026.
Applicants intending to use eCTD v4.0 should ensure that their systems, processes, and tools support the EU technical requirements, including the published validation criteria and controlled vocabularies. These documents and related guidance are available on the EMA eSubmission website. An additional practical guidance for EU eCTD v4.0 (for CAPs MAA) is now published on the same page.
EMA will provide updates on further steps in the eCTD v4.0 implementation roadmap.
The introduction of eCTD v4.0 represents a significant step towards a more harmonised and efficient electronic submission environment, offering enhanced metadata structures, improved lifecycle management, and greater interoperability with global regulatory authorities.
10-12-2025
EU eCTD v4.0 - Forward compatibility pilot for CAPs to be announced in 2026
EMA is pleased to announce that we are planning to start the Pilot Phase 3 on Forward Compatibility for the Centrally Authorised Products in 2026.
We strongly encourage all eCTD v4.0 tool vendors to ensure that their tools are ready to support Forward Compatibility in early 2026.
Further information and details how to participate will be published on the eSubmission website in Q1 2026.
AND
EU eCTD v4.0 - Validation criteria v1.1 - open for comments
The eCTD v4.0 EU Validation Criteria is under review. The draft updated validation criteria v1.1 is now available for your comments. Please send us your feedback via email to eCTD4consultation@ema.europa.eu by 31st December 2025.
04-11-2025
eCTD v4.0 - EU Controlled Vocabularies v3 now available
A new version of the EU eCTD v4.0 controlled vocabularies (v3) is
now available, in both excel
(.xlsx) and
genericode
(.xml) formats.
The changes compared to the previous version are highlighted in the excel
document, and marked with new/updated, accordingly. Please note that
previous versions of the EU controlled vocabularies are still valid.
A "Documentation" section is now available at the end of this page, containing the relevant
guidance, vocabularies, validation criteria for EU eCTD v4.0.
16-09-2025
eCTD v4.0 technical pilot phase 2 update
EMA is pleased to announce that the phase 2 of the eCTD v4.0 EU Technical pilot (CAPs)
continues and is progressing well. We are receiving submissions and useful findings have
been made. The participants should note that we are extending the phase
2 until the end of September 2025 to ensure that there is time to
receive and analyse further packages following important fixes into the review tool.
We would also like to highlight to the users the importance of using priority numbers
and careful use of keywords to ensure that sections,
such as 1.3 are not displayed multiple times.
26-08-2025
eCTD v4.0- Controlled Vocabularies v2 (genericode format) now available
The genericode format of the EU eCTD v4.0 controlled vocabularies (v2)
(published on the 8th of August in excel format only) is now available.
A new version (v3) will be published in the upcoming weeks, containing new terms
and corrections of existing terms.
AND
eCTD v4.0 technical pilot phase 2 update
EMA is pleased to announce that the phase 2 of the eCTD v4.0 EU Technical pilot (CAPs)
is ongoing, and progressing as planned.
The participants should take note that "multiple pack sizes" (Scenario 1)
is not meant to be reflected in the structure of the submissionUnit.xml, but it is simply related to
the content (the documents) of the test applications.
08-08-2025
eCTD v4.0 - Controlled Vocabularies v2 (.xlsx format) now available
A new version of the
EU eCTD v4.0 controlled vocabularies (v2)
is now available, in excel (.xlsx) format.
The genericode (.xml) format will be published soon.
The few changes compared to the previous version are highlighted in the document, and marked with new/updated, accordingly.
This version of the controlled vocabularies contains the new OID of the Implementation guide (1.3), which will also be published in the upcoming weeks.
01-08-2025
EU eCTD v4.0 validation criteria first version published
The EMA eCTD team and the EU eCTD v4.0 Subject Matter Experts are pleased to announce
that a draft EU eCTD v4.0 validation criteria is now available
here.
The list is a combination of rules existing in eCTD 3.2.2 (adapted to the new standard,
where necessary), and new rules, extracted from the eCTD v4.0 specification, mainly
related to the schema (for example mandatory elements and attributes).
The EU eCTD v4.0 validation criteria complements the latest
eCTD v4.0 ICH validation criteria.
The list is focused on the centrally authorised products (CAPs) and will be further
edited to contain rules for MRPs, DCPs and NAPs
The eCTD tool vendors are encouraged to start implementing the rules, in preparation for
the optional use of eCTD v4.0 for CAPs, planned for late Q4 2025. The scope of the
optional use and the actual start date depend on the results of the ongoing eCTD v4.0
technical pilot, and will be communicated in Q4.
For any concerns, recommendations or questions, please send an email to
ectd4consultation@ema.europa.eu. The EMA team, together with the EU eCTD v4.0 Subject
Matter Experts, will centralise and analyse the requests, and if considered valid, will
include them in the following version of the validation criteria.
30-06-2025
eCTD v4.0 Technical Pilot - Step 2 started
The electronic Common Technical Document (eCTD) v4.0 team is pleased to launch Step
2 of the eCTD v4.0 Technical Pilot, for Centrally Authorised Products (CAP).
Please note that the participation is very limited and the participants will be selected
based on the readiness and compliance with the scenarios and focus described below.
We would like to invite interested MAHs to provide details of their proposed products,
scenarios and very importantly the name of the eCTD v4.0 tool, by 15 July by email to eCTD4consultation@ema.europa.eu.
Upon confirmation from EMA will send details on how the test packages can be submitted
Important notes
- all the test submissions from Step 2 will be processed in an EMA
Test
environment and the submission channel will not be EMA Gateway
- a first draft of the validation criteria will be published in the
upcoming weeks, and the initial test submissions of Step 2 will not go through a
“standard” validation
The main scenarios below will be followed, but please send your proposals for
different/new scenarios when expressing your interest to participate:
- Scenario 1. Initial MAA (sequence 1); EMA will
prioritise for processing
those MAHs who can resubmit sequence 1 for an existing/already authorised CAP
(previously created in eCTD 3.2.2, sequence 0000, even if currently is not a
valid
product anymore), and now re-created/converted (fully or partially) in eCTD
v4.0.
Focus on:
- controlled vocabularies
- different file formats
- multiple pack sizes, manufacturers
- Scenario 2. Initial MAA (sequence 1) for a
Duplicate product sent in scenario 1 (the Duplicate product can be a mock
product; this will facilitate early testing of Grouped submissions
functionality, which is planned for Step 3)
Focus on:
- Document reuse
- different file formats
- Scenario 3. Validation responses (starting with
sequence 2), responses
Focus on:
- Document lifecycle management (replace context of use (one to
one, one to many and many to one), delete)
- Updating keywords, priority numbers, and document titles
- Regulatory activity (Submission and related sequences for 1
regulatory activity)
- Scenario 4. Post authorisation activities
(different procedure types and multiple sequences related to the products sent
in sequence 1)
Focus on:
- Document lifecycle management (replace context of use (one to
one, one to many and many to one), delete)
- Updating keywords, priority numbers, and document titles
- Parallel regulatory activities
In Step 2 (the planned initial duration of this step is 2 months: 15 July – 15
September, subject to extension if necessary) of the eCTD v4.0 of the technical pilot
there will be no focus on Grouped submissions functionality, forward compatibility.
These will be tested in Step 3, planned for late Q3 2025.
Please note: all the communication will take place over email and
depending on the
number of test packages received and EMA eCTD v4.0 team availability, there might be a
delay in the response time.
14-01-2025
Updated version of the "EU eCTD v4.0 Controlled Vocabularies" (.xml format)
An updated version of the EU eCTD v4.0 Controlled Vocabularies is now available
here.
The updated package contains the missing list (territorial authority) and the unused list was removed (dosage form category).
12-12-2024
EU eCTD v4.0 Controlled Vocabularies in .xml format now available
The package containing genericode (.xml) format for the first version of the EU
eCTD v4.0 Controlled Vocabularies is now available
here.
They are complementing the .xlsx version which was published in October.
03-12-2024
Call for Interest eCTD v4.0 Technical Pilot
The electronic Common Technical Document (eCTD) v4.0 is pleased to announce the launch
of the eCTD v4.0
Technical Pilot and invite interested eCTD tool vendors to participate in Step
1. A call for participation in
the following steps will be launched at a later stage.
If you have not yet expressed your interest, please send an email to eCTD4consultation@ema.europa.eu by 20
December 2024 to express your intention to participate. If approved, you will
receive a confirmation e-mail with further instructions.
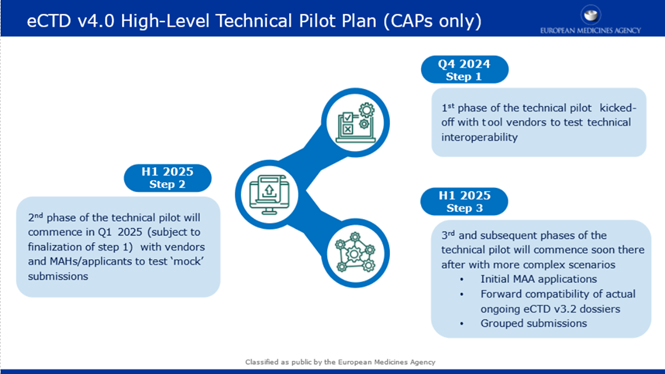
Step 1
- Participants: Tool vendors, EMA
- Timeline: Q4 2024 - Q1 2025
- Focus Area: Technical Interoperability
Step 2
- Participants: Tool vendors (potential collaboration with MAHs), EMA
- Timeline: Q1 2025 (Subject to finalisation of step 1) - TBD
- Focus Areas:
- Simple scenarios (mock submissions, single package/sequence)
- Document lifecycle management (e.g. replacements, updates)
- Handling of multiple file formats, pack sizes, manufacturers
- Review of controlled vocabularies (CV)
- Updating keywords, priority numbers, and document titles
- Document reuse
Step 3
- Participants: Tool vendors, MAHs, EMA
- Timeline: Subject to finalisation of step 2, TBD
- Focus Areas:
- Expansion of Step 2 scenarios with non-mock data
- Grouped submissions functionality, forward compatibility
- Parallel regulatory activities
PFor a high-level view, please refer to the attached pilot plan.
04-10-2024
EU eCTD v4.0 draft Implementation package now available
An updated draft version of the EU eCTD v4.0 implementation package is now available here.
The package contains the following:
- EU eCTD v4.0 Implementation Guide draft version 1.2 ; this version is focused on CAPs and the main changes since version 1.1 are reflected in the "Document change history" of the file
- EU eCTD v4.0 Controlled Vocabularies, in .xlsx format (version 1); the genericode files (.xml format) will be published in the following weeks
- EU eCTD v4.0 Accepted file formats
An upcoming updated package (containing the genericode files for the controlled vocabularies and the validation criteria) will be made available in the coming period.
The package is meant for eCTD tool vendors to access the new format of the Controlled Vocabularies and the new OIDs, as well as to be notified by the changes in the EU eCTD v4.0 Implementation Guide (version 1.2).
According to the EU eCTD v4.0 implementation timeline, a technical pilot for eCTD v4.0 in EU (focused on CAPs) is planned to start by the end of 2024, and more details will be published on the eSubmission website. If you are an eCTD tool vendor, have already developed eCTD v4.0 capabilities in the tool and are interested in participating in the EU eCTD v4.0 pilot, follow the eSubmission website for the upcoming announcement, or send your intention to join the pilot at ectd4consultation@ema.europa.eu.
13-06-2024
ICH eCTD v4.0 package updated
The ICH eCTD v4.0 package has been updated in response to the change requests and/or discussion within the EWG after its initial release. There has been number of updates to the ICH IG and both the Support Documentation and the Orientation Material presentations have been updated. Additionally, as of May 2024, the Implementation Guide and Controlled Vocabulary documents were split into two different packages to enable Controlled Vocabulary Versioning.
More information can be found on the ICH Official web site: ICH.
AND
ICH Call for Vendor participation now open
A call for vendor participation is now published on the ICH Official website.
If you are an eCTD v4.0 Tool Vendor and would like to join the eCTD Tool Vendor Group to discuss eCTD v4.0 implementation, please send the following information listed below to ICHM8Vendors@ich.org:
- First Name;
- Last Name;
- Email;
- Company Name;
Please note that your information will be shared as needed with Representatives of ICH Members and Observers involved with ICH eCTD activities.
The Vendor Group kick-off meeting will be held on 17 July 2024, at 7.00 A.M. ET.
The vendor group is solely intended to facilitate collaborative discussion on the eCTD v4.0 Implementation Specification and timelines.
More information can be found on the ICH Official web site: ICH.
15-03-2024
Register for the eCTD v4.0 Vendor Workshop on 27 March 2024
The electronic Common Technical Document (eCTD) is the standard norm for industry submissions for over 20 years and currently implemented by regulators and industry in version 3.2.2. Version 4.0 of eCTD was published in December 2015 with its most recent Implementation Package v1.5 endorsed at the May 2022 ICH meeting.
This workshop, organised and facilitated by the eCTD v4.0 SME Group (EMA, NCAs and Industry), is intended for all eCTD Vendors that are interested in better understanding changes to the EU regional eCTD Specification. The event aims at fostering a discussion around the key sections of the new version of the Implementation Guide, the technical understanding of the implementation of the new standard, including the grouped submissions, document re-use and controlled vocabularies. At the moment, the focus will be on the Centralised Procedures only.
Moreover, during the workshop, the scope and timeline of the pilot, as well as the timeline for the updates of the Implementation Guide, will be discussed.
This session also offers an invaluable opportunity for participants to ask questions and provide feedback on pertinent matters.
Register for this workshop.
After registration you will receive a confirmation e-mail with the link and password to access the workshop. Please make sure to save those to be able to participate to the workshop.
14-03-2024
eCTD v4.0 EU M1 Implementation Guide - draft Version 1.1 now available
A new draft version of the eCTD v4.0 EU M1 Implementation Guide is now available here for consultation. Future versions (together with EU Controlled Vocabularies and other annexes) will be published and announced on the eSubmission website.
21-12-2023
eCTD v4.0 Vendor Workshop Survey
EMA, together with the eCTD v4.0 EU Subject Matter Experts group, is planning a Vendor Workshop in Q1 2024. To assess the availability, interest and further topics for discussion, the Vendors are invited to complete the eCTD v4.0 Vendor Workshop survey by end of day 15 February 2024.
11-07-2023
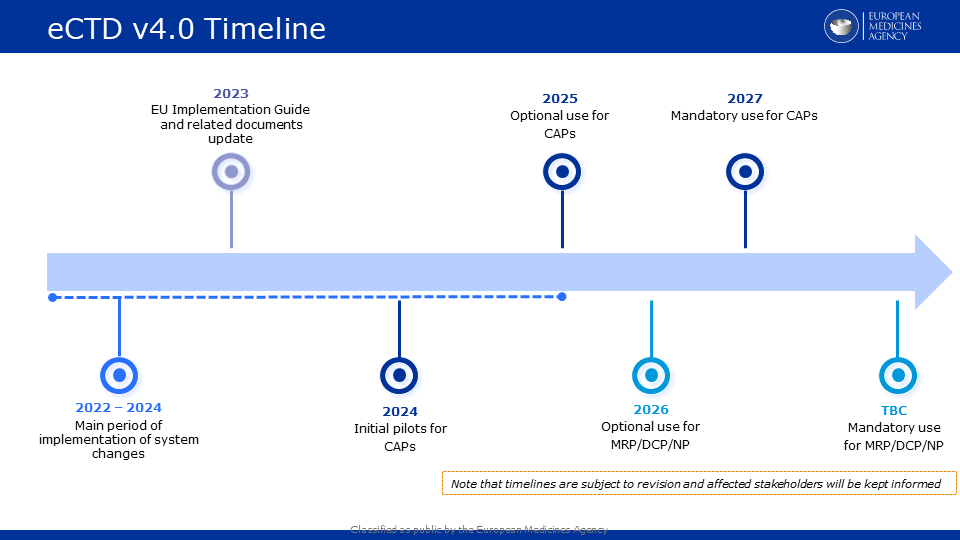
Updated draft eCTD v4.0 implementation timeline for EU now available
Please see below an updated draft timeline for the implementation of eCTD v4.0 in the EU.
EU eCTD v4.0 documentation:
|

